MicroRNA-802 plays a tumour suppressive role in tongue squamous cell carcinoma through directly _targeting MAP2K4
- PMID: 28319306
- PMCID: PMC6529076
- DOI: 10.1111/cpr.12336
MicroRNA-802 plays a tumour suppressive role in tongue squamous cell carcinoma through directly _targeting MAP2K4
Abstract
Objectives: Tongue squamous cell carcinoma (TSCC) is the most common oral tumours. MicroRNAs play crucial roles in many cell processes including cell viability, development, apoptosis, migration and invasion. The role of miR-802 in the TSCC is still unknown.
Materials and methods: The miR-802 expression in TSCC tissues and cell lines was determined by quantitative real-time polymerase chain reaction. CCK-8 assay was performed to measure the cell viability, while the cell invasion assay was used to determine the cell invasion. Dual-luciferase reporter and western blot were used to confirm the potential _target gene of miR-802.
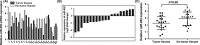
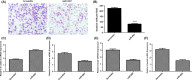
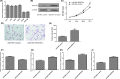
Results: In our study, we demonstrated that miR-802 expression was downregulated in TSCC tissues and cell lines. Elevated expression of miR-802 suppressed the TSCC cell viability and invasion. Moreover, enforced expression of miR-802 increased the expression of E-cadherin, while suppressed the expression of N-cadherin, Snail and Vimentin in the TSCC cell. In addition, we identified the mitogen-activated protein kinase 4 (MAP2K4) as a direct _target gene of miR-802 in the TSCC cell. We also demonstrated that the expression of MAP2K4 was higher in the TSCC tissues than that in the adjacent normal tissues. Furthermore, the expression level of MAP2K4 was inversely associated with the expression of miR-802 in TSCC tissues. We also demonstrated that the MAP2K4 expression was upregulated in TSCC cell lines. Elevated expression of miR-802 inhibited TSCC cell viability and invasion through inhibiting MAP2K4 expression.
Conclusions: Our data revealed that miR-802 played as a tumour suppressor gene and might act as a therapeutic _target in TSCC patients.
Keywords: MAP2K4; miR-802; microRNAs; tongue squamous cell carcinoma.
© 2017 John Wiley & Sons Ltd.
Figures
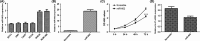

Similar articles
-
Hsa_circ_0081069 facilitates tongue squamous cell carcinoma progression by modulating MAP2K4 expression via miR-634.Odontology. 2023 Apr;111(2):474-486. doi: 10.1007/s10266-022-00746-0. Epub 2022 Oct 1. Odontology. 2023. PMID: 36181561
-
MicroRNA-137 suppresses tongue squamous carcinoma cell proliferation, migration and invasion.Cell Prolif. 2016 Oct;49(5):628-35. doi: 10.1111/cpr.12287. Epub 2016 Aug 30. Cell Prolif. 2016. PMID: 27571935 Free PMC article.
-
miR-34a inhibits migration and invasion of tongue squamous cell carcinoma via _targeting MMP9 and MMP14.PLoS One. 2014 Sep 30;9(9):e108435. doi: 10.1371/journal.pone.0108435. eCollection 2014. PLoS One. 2014. PMID: 25268950 Free PMC article.
-
MicroRNA expression and its implications for diagnosis and therapy of tongue squamous cell carcinoma.J Cell Mol Med. 2016 Jan;20(1):10-6. doi: 10.1111/jcmm.12650. Epub 2015 Oct 24. J Cell Mol Med. 2016. PMID: 26498914 Free PMC article. Review.
-
MicroRNAs in human tongue squamous cell carcinoma: From pathogenesis to therapeutic implications.Oral Oncol. 2017 Apr;67:124-130. doi: 10.1016/j.oraloncology.2017.02.015. Epub 2017 Feb 27. Oral Oncol. 2017. PMID: 28351566 Review.
Cited by
-
TRG-AS1 is a potent driver of oncogenicity of tongue squamous cell carcinoma through microRNA-543/Yes-associated protein 1 axis regulation.Cell Cycle. 2020 Aug;19(15):1969-1982. doi: 10.1080/15384101.2020.1786622. Epub 2020 Jul 2. Cell Cycle. 2020. Update in: Cell Cycle. 2021 Dec;20(24):2671. doi: 10.1080/15384101.2021.1973198 Retraction in: Cell Cycle. 2023 Mar-Mar;22(6):737. doi: 10.1080/15384101.2023.2171198 PMID: 32615889 Free PMC article. Updated. Retracted.
-
miR‑802 inhibits the epithelial‑mesenchymal transition, migration and invasion of cervical cancer by regulating BTF3.Mol Med Rep. 2020 Sep;22(3):1883-1891. doi: 10.3892/mmr.2020.11267. Epub 2020 Jun 23. Mol Med Rep. 2020. PMID: 32582971 Free PMC article.
-
MicroRNA-488 inhibits tongue squamous carcinoma cell invasion and EMT by directly _targeting ATF3.Cell Mol Biol Lett. 2018 Jun 18;23:28. doi: 10.1186/s11658-018-0094-0. eCollection 2018. Cell Mol Biol Lett. 2018. Retraction in: Cell Mol Biol Lett. 2022 Dec 27;27(1):112. doi: 10.1186/s11658-022-00414-9 PMID: 29946339 Free PMC article. Retracted.
-
Overexpression of Chromosome 21 miRNAs May Affect Mitochondrial Function in the Hearts of Down Syndrome Fetuses.Int J Genomics. 2017;2017:8737649. doi: 10.1155/2017/8737649. Epub 2017 Sep 5. Int J Genomics. 2017. PMID: 29057256 Free PMC article.
-
MicroRNA-802 Suppresses Tumorigenesis of Colorectal Cancer via Regulating UBN2.Cancer Manag Res. 2020 Nov 4;12:11219-11230. doi: 10.2147/CMAR.S267345. eCollection 2020. Cancer Manag Res. 2020. PMID: 33177873 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

