Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and _targets for Intervention
- PMID: 28475116
- PMCID: PMC5454897
- DOI: 10.3390/ijms18050984
Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and _targets for Intervention
Abstract
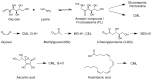
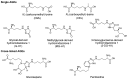
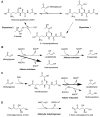
Advanced glycation end-products (AGEs) are non-enzymatic protein and amino acid adducts as well as DNA adducts which form from dicarbonyls and glucose. AGE formation is enhanced in diabetes and is associated with the development of diabetic complications. In the current review, we discuss mechanisms that lead to enhanced AGE levels in the context of diabetes and diabetic complications. The methylglyoxal-detoxifying glyoxalase system as well as alternative pathways of AGE detoxification are summarized. Therapeutic approaches to interfere with different pathways of AGE formation are presented.
Keywords: 3-deoxyglucosone; advanced glycation end-products; aldose reductase; diabetes; glyoxal; glyoxalase; methylglyoxal.
Conflict of interest statement
Sebastian Brings, Thomas Fleming and Peter P. Nawroth are named as inventors in a pending patent that discloses the use of scavenger peptides for the treatment of MG induced complications.
Figures

Similar articles
-
Alpha-Synuclein Glycation and the Action of Anti-Diabetic Agents in Parkinson's Disease.J Parkinsons Dis. 2018;8(1):33-43. doi: 10.3233/JPD-171285. J Parkinsons Dis. 2018. PMID: 29480231 Free PMC article. Review.
-
Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation.Biochem Pharmacol. 1999 Dec 1;58(11):1765-73. doi: 10.1016/s0006-2952(99)00263-4. Biochem Pharmacol. 1999. PMID: 10571251
-
Protein Repair from Glycation by Glyoxals by the DJ-1 Family Maillard Deglycases.Adv Exp Med Biol. 2017;1037:133-147. doi: 10.1007/978-981-10-6583-5_9. Adv Exp Med Biol. 2017. PMID: 29147907
-
_targeting advanced glycation with pharmaceutical agents: where are we now?Glycoconj J. 2016 Aug;33(4):653-70. doi: 10.1007/s10719-016-9691-1. Epub 2016 Jul 9. Glycoconj J. 2016. PMID: 27392438 Review.
-
Metformin inhibition of glycation processes.Diabetes Metab. 2003 Sep;29(4 Pt 2):6S95-103. doi: 10.1016/s1262-3636(03)72793-1. Diabetes Metab. 2003. PMID: 14502106 Review.
Cited by
-
Glycation by glyoxal leads to profound changes in the behavior of dermal fibroblasts.BMJ Open Diabetes Res Care. 2021 Apr;9(1):e002091. doi: 10.1136/bmjdrc-2020-002091. BMJ Open Diabetes Res Care. 2021. PMID: 33903117 Free PMC article.
-
Alpha-Synuclein Glycation and the Action of Anti-Diabetic Agents in Parkinson's Disease.J Parkinsons Dis. 2018;8(1):33-43. doi: 10.3233/JPD-171285. J Parkinsons Dis. 2018. PMID: 29480231 Free PMC article. Review.
-
Copper, Iron, Selenium and Lipo-Glycemic Dysmetabolism in Alzheimer's Disease.Int J Mol Sci. 2021 Aug 31;22(17):9461. doi: 10.3390/ijms22179461. Int J Mol Sci. 2021. PMID: 34502369 Free PMC article. Review.
-
Dieckol, Derived from the Edible Brown Algae Ecklonia cava, Attenuates Methylglyoxal-Associated Diabetic Nephropathy by Suppressing AGE-RAGE Interaction.Antioxidants (Basel). 2023 Feb 27;12(3):593. doi: 10.3390/antiox12030593. Antioxidants (Basel). 2023. PMID: 36978839 Free PMC article.
-
Emerging and multifaceted potential contributions of polyphenols in the management of type 2 diabetes mellitus.World J Diabetes. 2024 Feb 15;15(2):154-169. doi: 10.4239/wjd.v15.i2.154. World J Diabetes. 2024. PMID: 38464365 Free PMC article. Review.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 7th ed. International Diabetes Federation; Brussels, Belgium: 2015.
-
- Genuth S., Sun W., Cleary P., Gao X., Sell D.R., Lachin J., Group D.E.R., Monnier V.M. Skin Advanced Glycation End Products Glucosepane and Methylglyoxal Hydroimidazolone Are Independently Associated with Long-Term Microvascular Complication Progression of Type 1 Diabetes. Diabetes. 2015;64:266–278. doi: 10.2337/db14-0215. - DOI - PMC - PubMed
-
- Genuth S., Sun W., Cleary P., Sell D.R., Dahms W., Malone J., Sivitz W., Monnier V.M. Glycation and Carboxymethyllysine Levels in Skin Collagen Predict the Risk of Future 10-Year Progression of Diabetic Retinopathy and Nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications Participants with Type 1 Diabetes. Diabetes. 2005;54:3103–3111. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

