The Role of Platelet-Derived ADP and ATP in Promoting Pancreatic Cancer Cell Survival and Gemcitabine Resistance
- PMID: 29064388
- PMCID: PMC5664081
- DOI: 10.3390/cancers9100142
The Role of Platelet-Derived ADP and ATP in Promoting Pancreatic Cancer Cell Survival and Gemcitabine Resistance
Abstract
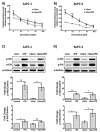
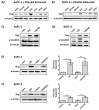
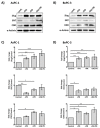
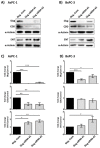
Platelets have been demonstrated to be vital in cancer epithelial-mesenchymal transition (EMT), an important step in metastasis. Markers of EMT are associated with chemotherapy resistance. However, the association between the development of chemoresistance, EMT, and the contribution of platelets to the process, is still unclear. Here we report that platelets regulate the expression of (1) human equilibrative nucleoside transporter 1 (hENT1) and (2) cytidine deaminase (CDD), markers of gemcitabine resistance in pancreatic cancer. Human ENT1 (hENT1) is known to enable cellular uptake of gemcitabine while CDD deactivates gemcitabine. Knockdown experiments demonstrate that Slug, a mesenchymal transcriptional factor known to be upregulated during EMT, regulates the expression of hENT1 and CDD. Furthermore, we demonstrate that platelet-derived ADP and ATP regulate Slug and CDD expression in pancreatic cancer cells. Finally, we demonstrate that pancreatic cancer cells express the purinergic receptor P2Y12, an ADP receptor found mainly on platelets. Thus ticagrelor, a P2Y12 inhibitor, was used to examine the potential therapeutic effect of an ADP receptor antagonist on cancer cells. Our data indicate that ticagrelor negated the survival signals initiated in cancer cells by platelet-derived ADP and ATP. In conclusion, our results demonstrate a novel role of platelets in modulating chemoresistance in pancreatic cancer. Moreover, we propose ADP/ATP receptors as additional potential drug _targets for treatment of pancreatic cancer.
Keywords: ADP; ATP; gemcitabine; pancreatic cancer; platelets.
Conflict of interest statement
The authors report no conflict of interest.
Figures

Similar articles
-
Antiplatelet Drug Ticagrelor Enhances Chemotherapeutic Efficacy by _targeting the Novel P2Y12-AKT Pathway in Pancreatic Cancer Cells.Cancers (Basel). 2020 Jan 20;12(1):250. doi: 10.3390/cancers12010250. Cancers (Basel). 2020. PMID: 31968611 Free PMC article.
-
EMT-Induced Gemcitabine Resistance in Pancreatic Cancer Involves the Functional Loss of Equilibrative Nucleoside Transporter 1.Mol Cancer Ther. 2021 Feb;20(2):410-422. doi: 10.1158/1535-7163.MCT-20-0316. Epub 2020 Dec 9. Mol Cancer Ther. 2021. PMID: 33298588 Free PMC article.
-
Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization.Oncol Lett. 2017 Nov;14(5):5400-5408. doi: 10.3892/ol.2017.6836. Epub 2017 Aug 28. Oncol Lett. 2017. PMID: 29098031 Free PMC article.
-
P2Y12 Receptors in Tumorigenesis and Metastasis.Front Pharmacol. 2018 Feb 2;9:66. doi: 10.3389/fphar.2018.00066. eCollection 2018. Front Pharmacol. 2018. PMID: 29456511 Free PMC article. Review.
-
Attempts to remodel the pathways of gemcitabine metabolism: Recent approaches to overcoming tumours with acquired chemoresistance.Cancer Drug Resist. 2020 Oct 12;3(4):819-831. doi: 10.20517/cdr.2020.39. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582220 Free PMC article. Review.
Cited by
-
The Role of Platelets in the Tumor-Microenvironment and the Drug Resistance of Cancer Cells.Cancers (Basel). 2019 Feb 19;11(2):240. doi: 10.3390/cancers11020240. Cancers (Basel). 2019. PMID: 30791448 Free PMC article. Review.
-
Mitochondrial Protein UQCRC1 is Oncogenic and a Potential Therapeutic _target for Pancreatic Cancer.Theranostics. 2020 Jan 12;10(5):2141-2157. doi: 10.7150/thno.38704. eCollection 2020. Theranostics. 2020. PMID: 32089737 Free PMC article.
-
Barriers and opportunities for gemcitabine in pancreatic cancer therapy.Am J Physiol Cell Physiol. 2023 Feb 1;324(2):C540-C552. doi: 10.1152/ajpcell.00331.2022. Epub 2022 Dec 26. Am J Physiol Cell Physiol. 2023. PMID: 36571444 Free PMC article. Review.
-
Antiplatelet Drug Ticagrelor Enhances Chemotherapeutic Efficacy by _targeting the Novel P2Y12-AKT Pathway in Pancreatic Cancer Cells.Cancers (Basel). 2020 Jan 20;12(1):250. doi: 10.3390/cancers12010250. Cancers (Basel). 2020. PMID: 31968611 Free PMC article.
-
Purinergic signaling: Diverse effects and therapeutic potential in cancer.Front Oncol. 2023 Jan 18;13:1058371. doi: 10.3389/fonc.2023.1058371. eCollection 2023. Front Oncol. 2023. PMID: 36741002 Free PMC article. Review.
References
-
- Weiler H. A platelet cloak for tumor cells. Blood. 2005;105:5–6. doi: 10.1182/blood-2004-10-3868. - DOI
-
- Palumbo J.S., Talmage K.E., Massari J.V., La Jeunesse C.M., Flick M.J., Kombrinck K.W., Jirouskova M., Degen J.L. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

