Age and fecal microbial strain-specific differences in patients with spondyloarthritis
- PMID: 29382366
- PMCID: PMC5791354
- DOI: 10.1186/s13075-018-1510-6
Age and fecal microbial strain-specific differences in patients with spondyloarthritis
Abstract
Background: Prior studies have demonstrated abnormalities in the composition of the gastrointestinal microbiota in pediatric and adult patients with spondyloarthritis (SpA). In particular, diminished fecal abundance of Faecalibacterium prausnitzii and abnormalities in both directions in the abundance of the Bacteroides genus have been identified.
Methods: We obtained fecal specimens from 30 children with treatment-naïve enthesitis-related arthritis (ERA) and 19 healthy controls, as well as specimens from 11 adult patients with longstanding SpA and 10 adult healthy controls. All of the samples underwent sequencing of the 16S ribosomal DNA. A subset of the pediatric fecal samples was subjected to shotgun metagenomics sequencing.
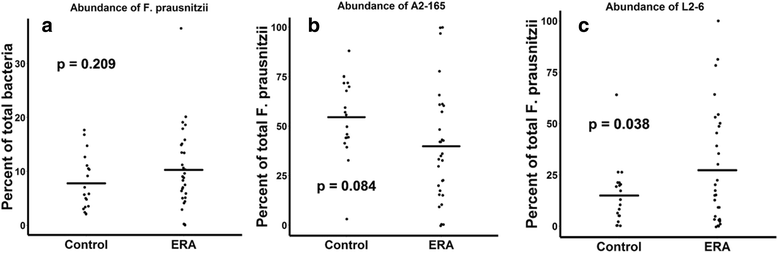
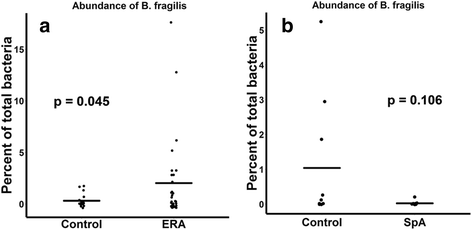
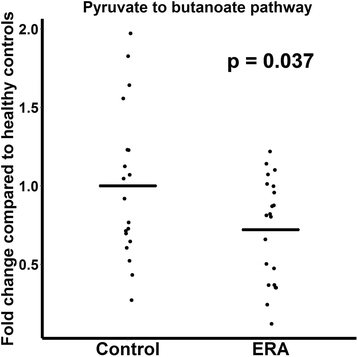
Results: ERA patients had decreased abundance of the anti-inflammatory F. prausnitzii A2-165 strain (41 ± 28% versus 54 ± 20% of all sequences matching F. prausnitzii, p = 0.084) and an increased abundance of the control F. prausnitzii L2/6 strain (28 ± 28% versus 15 ± 15%, p = 0.038). Similar trends were observed in adults with longstanding SpA (n = 11) and controls (n = 10). In contrast, the fecal abundance of Bacteroides fragilis was increased in ERA subjects (2.0 ± 4.0% versus 0.45 ± 0.7% of all sequences, p = 0.045), yet was diminished in adult subjects (0.2 ± % versus 1.0 ± % of all sequences, p = 0.106). Shotgun metagenomics sequencing of the fecal DNA in the pediatric subjects revealed diminished coverage of the butanoate pathway (abundance normalized to controls of 1 ± 0.48 versus 0.72 ± 0.33 in ERA, p = 0.037).
Conclusions: The anti-inflammatory F. prausnitzii A2-165 strain appears to be depleted in both pediatric and adult SpA. In contrast, B. fragilis may be depleted in adult disease yet abundant in pediatric SpA, suggesting developmental effects on the immune system.
Keywords: Bacteroides; Faecalibacterium; Microbiota; Sequencing; Spondyloarthritis.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Office of the IRB at UAB as well as at the respective study sites: Children’s Hospital of Philadelphia, Hackensack University Medical Center, Children’s Hospital Boston, Connecticut Children’s Medical Center, Nationwide Children’s Hospital, University of Texas at Southwestern Medical Center (which governs Texas Scottish Rite Hospital), University of Louisville, and Children’s Hospital of LA. Informed consent and assent as appropriate were obtained from all study subjects and/or their guardians as per local regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis.Arthritis Res Ther. 2014 Nov 30;16(6):486. doi: 10.1186/s13075-014-0486-0. Arthritis Res Ther. 2014. PMID: 25434931 Free PMC article.
-
Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease.Digestion. 2016;93(1):59-65. doi: 10.1159/000441768. Epub 2016 Jan 14. Digestion. 2016. PMID: 26789999
-
Fecal metabolomics in pediatric spondyloarthritis implicate decreased metabolic diversity and altered tryptophan metabolism as pathogenic factors.Genes Immun. 2016 Dec;17(7):400-405. doi: 10.1038/gene.2016.38. Epub 2016 Oct 27. Genes Immun. 2016. PMID: 27786174 Free PMC article.
-
The intestinal microbiome in spondyloarthritis.Curr Opin Rheumatol. 2015 Jul;27(4):319-25. doi: 10.1097/BOR.0000000000000187. Curr Opin Rheumatol. 2015. PMID: 26002022 Free PMC article. Review.
-
The microbiota in pediatric rheumatic disease: epiphenomenon or therapeutic _target?Curr Opin Rheumatol. 2016 Sep;28(5):537-43. doi: 10.1097/BOR.0000000000000312. Curr Opin Rheumatol. 2016. PMID: 27286235 Free PMC article. Review.
Cited by
-
Spondyloarthritis: How far are we from precision medicine?Front Med (Lausanne). 2022 Sep 8;9:988532. doi: 10.3389/fmed.2022.988532. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36160128 Free PMC article. Review.
-
Similarities and Differences Between Juvenile and Adult Spondyloarthropathies.Front Med (Lausanne). 2021 May 31;8:681621. doi: 10.3389/fmed.2021.681621. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34136509 Free PMC article. Review.
-
Outcomes in Juvenile-Onset Spondyloarthritis.Front Med (Lausanne). 2021 May 28;8:680916. doi: 10.3389/fmed.2021.680916. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34124112 Free PMC article. Review.
-
Recent Updates in Juvenile Spondyloarthritis.Rheum Dis Clin North Am. 2021 Nov;47(4):565-583. doi: 10.1016/j.rdc.2021.07.001. Epub 2021 Aug 21. Rheum Dis Clin North Am. 2021. PMID: 34635292 Free PMC article. Review.
-
A Pilot Study Investigating Faecal Microbiota After Two Dietary Interventions in Children with Juvenile Idiopathic Arthritis.Curr Microbiol. 2022 Jun 7;79(7):215. doi: 10.1007/s00284-022-02899-1. Curr Microbiol. 2022. PMID: 35672613 Free PMC article.
References
-
- van Sommeren S, Janse M, Karjalainen J, Fehrmann R, Franke L, Fu J, et al. Extraintestinal manifestations and complications in inflammatory bowel disease: from shared genetics to shared biological pathways. Inflamm Bowel Dis. 2014;20(6):987–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

