The novel dithiocarbamate, DpdtC suppresses HER2-overexpressed cancer cells by up-regulating NDRG1 via inactivation of HER2-ERK 1/2 signaling
- PMID: 29467385
- PMCID: PMC5821706
- DOI: 10.1038/s41598-018-21768-1
The novel dithiocarbamate, DpdtC suppresses HER2-overexpressed cancer cells by up-regulating NDRG1 via inactivation of HER2-ERK 1/2 signaling
Abstract
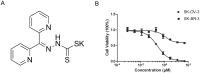
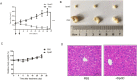
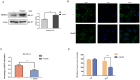
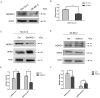
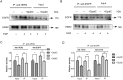
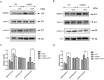
Dithiocarbamate has been tested for its effective anti-tumor activity, but the underlying mechanism remains unclear. We previously prepared a novel diththiocarbamate derivative, DpdtC with an ability of catalase inhibition. Here, we for the first time investigated the growth inhibition effects of DpdtC on HER2-amplified cancer cells and elucidated its mechanism of action. Results showed that DpdtC exerted the potent anti-tumor effects against HER2-overexpressed SK-OV-3 and SK-BR-3 cells, especially on SK-OV-3 cells with a higher NDRG1 level, which was also confirmed in the SK-OV-3 xenograft model. Interestingly, we observed that NDRG1 was up-regulated, while membrane expression of HER2 was regressed in SK-OV-3 cells upon DpdtC treatment. In agreement, silencing endogenous NDRG1 also increased the expression of HER2 in SK-OV-3 cells, while overexpressing NDRG1 decreased HER2 expression in SK-BR-3 cells. Furthermore, our results showed the formation of the EGFR/HER2 heterodimer was attenuated and phosphorylation of ERK1/2 was inhibited in SK-OV-3 cells when treated with DpdtC. Collectively, these observations demonstrated that NDRG1 plays an important role in mediating the inhibition effects of DpdtC in HER2-overexpressed cancer cells via selective _targeting of the HER2-ERK1/2 pathway. Hence, our investigation suggests that up-regulation of NDRG1 by DpdtC is a promising therapeutic approach in HER2-overexpressed cancers.
Conflict of interest statement
The authors declare no competing interests.
Figures
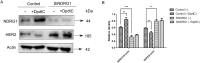
Similar articles
-
The Metastasis Suppressor, N-MYC Downstream-regulated Gene-1 (NDRG1), Down-regulates the ErbB Family of Receptors to Inhibit Downstream Oncogenic Signaling Pathways.J Biol Chem. 2016 Jan 15;291(3):1029-52. doi: 10.1074/jbc.M115.689653. Epub 2015 Nov 3. J Biol Chem. 2016. PMID: 26534963 Free PMC article.
-
SV40 T/t-common polypeptide inhibits angiogenesis and growth of HER2-overexpressing human ovarian cancer.Cancer Gene Ther. 2011 Dec;18(12):859-70. doi: 10.1038/cgt.2011.55. Epub 2011 Aug 26. Cancer Gene Ther. 2011. PMID: 21869825
-
Endophilin A2 promotes HER2 internalization and sensitivity to trastuzumab-based therapy in HER2-positive breast cancers.Breast Cancer Res. 2017 Oct 3;19(1):110. doi: 10.1186/s13058-017-0900-z. Breast Cancer Res. 2017. PMID: 28974266 Free PMC article.
-
The role of the NDRG1 in the pathogenesis and treatment of breast cancer.Biochim Biophys Acta Rev Cancer. 2023 May;1878(3):188871. doi: 10.1016/j.bbcan.2023.188871. Epub 2023 Feb 24. Biochim Biophys Acta Rev Cancer. 2023. PMID: 36841367 Review.
-
Interplay of the iron-regulated metastasis suppressor NDRG1 with epidermal growth factor receptor (EGFR) and oncogenic signaling.J Biol Chem. 2017 Aug 4;292(31):12772-12782. doi: 10.1074/jbc.R117.776393. Epub 2017 Jun 14. J Biol Chem. 2017. PMID: 28615452 Free PMC article. Review.
Cited by
-
A novel antitumor dithiocarbamate compound inhibits the EGFR/AKT signaling pathway and induces apoptosis in esophageal cancer cells.Oncol Lett. 2020 Jul;20(1):877-883. doi: 10.3892/ol.2020.11638. Epub 2020 May 18. Oncol Lett. 2020. PMID: 32566015 Free PMC article.
-
NDRG1 suppresses vasculogenic mimicry and tumor aggressiveness in gastric carcinoma.Oncol Lett. 2019 Sep;18(3):3003-3016. doi: 10.3892/ol.2019.10642. Epub 2019 Jul 22. Oncol Lett. 2019. PMID: 31452779 Free PMC article.
-
The revival of dithiocarbamates: from pesticides to innovative medical treatments.iScience. 2021 Jan 22;24(2):102092. doi: 10.1016/j.isci.2021.102092. eCollection 2021 Feb 19. iScience. 2021. PMID: 33598645 Free PMC article. Review.
-
Synthesis, and biological evaluation of EGFR/HER2-NAMPT conjugates for tumor treatment.Mol Divers. 2024 Aug;28(4):2617-2636. doi: 10.1007/s11030-023-10701-y. Epub 2023 Jul 23. Mol Divers. 2024. PMID: 37481750
-
Knockdown of NAA25 Suppresses Breast Cancer Progression by Regulating Apoptosis and Cell Cycle.Front Oncol. 2022 Jan 13;11:755267. doi: 10.3389/fonc.2021.755267. eCollection 2021. Front Oncol. 2022. PMID: 35096568 Free PMC article.
References
-
- Kovacevic Z, Chikhani S, Lovejoy DB, Richardson DR. Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: a new strategy for the treatment of pancreatic cancer. Mol Pharmacol. 2011;80:598–609. doi: 10.1124/mol.111.073627. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

