Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms
- PMID: 29945898
- PMCID: PMC6020109
- DOI: 10.1124/pr.117.015198
Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms
Erratum in
-
Correction to "Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms".Pharmacol Rev. 2018 Oct;70(4):879. doi: 10.1124/pr.116.015198err. Pharmacol Rev. 2018. PMID: 30282701 No abstract available.
Abstract
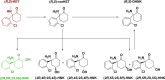
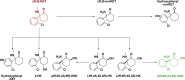
Ketamine, a racemic mixture consisting of (S)- and (R)-ketamine, has been in clinical use since 1970. Although best characterized for its dissociative anesthetic properties, ketamine also exerts analgesic, anti-inflammatory, and antidepressant actions. We provide a comprehensive review of these therapeutic uses, emphasizing drug dose, route of administration, and the time course of these effects. Dissociative, psychotomimetic, cognitive, and peripheral side effects associated with short-term or prolonged exposure, as well as recreational ketamine use, are also discussed. We further describe ketamine's pharmacokinetics, including its rapid and extensive metabolism to norketamine, dehydronorketamine, hydroxyketamine, and hydroxynorketamine (HNK) metabolites. Whereas the anesthetic and analgesic properties of ketamine are generally attributed to direct ketamine-induced inhibition of N-methyl-D-aspartate receptors, other putative lower-affinity pharmacological _targets of ketamine include, but are not limited to, γ-amynobutyric acid (GABA), dopamine, serotonin, sigma, opioid, and cholinergic receptors, as well as voltage-gated sodium and hyperpolarization-activated cyclic nucleotide-gated channels. We examine the evidence supporting the relevance of these _targets of ketamine and its metabolites to the clinical effects of the drug. Ketamine metabolites may have broader clinical relevance than was previously considered, given that HNK metabolites have antidepressant efficacy in preclinical studies. Overall, pharmacological _target deconvolution of ketamine and its metabolites will provide insight critical to the development of new pharmacotherapies that possess the desirable clinical effects of ketamine, but limit undesirable side effects.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Ketamine's dose related multiple mechanisms of actions: Dissociative anesthetic to rapid antidepressant.Behav Brain Res. 2020 Jul 15;390:112631. doi: 10.1016/j.bbr.2020.112631. Epub 2020 May 8. Behav Brain Res. 2020. PMID: 32437885 Review.
-
Mechanisms of ketamine action as an antidepressant.Mol Psychiatry. 2018 Apr;23(4):801-811. doi: 10.1038/mp.2017.255. Epub 2018 Mar 13. Mol Psychiatry. 2018. PMID: 29532791 Free PMC article. Review.
-
A Phase 1 Assessment of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of (2R,6R)-Hydroxynorketamine in Healthy Volunteers.Clin Pharmacol Ther. 2024 Nov;116(5):1314-1324. doi: 10.1002/cpt.3391. Epub 2024 Jul 25. Clin Pharmacol Ther. 2024. PMID: 39054770 Clinical Trial.
-
Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective.Psychiatry Clin Neurosci. 2019 Oct;73(10):613-627. doi: 10.1111/pcn.12902. Epub 2019 Jul 11. Psychiatry Clin Neurosci. 2019. PMID: 31215725 Free PMC article. Review.
-
Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine.Neuropharmacology. 2019 Jul 15;153:73-81. doi: 10.1016/j.neuropharm.2019.04.019. Epub 2019 Apr 20. Neuropharmacology. 2019. PMID: 31015046
Cited by
-
Unexpected consequences: A case of ketamine-induced seizure in procedural sedation.Turk J Emerg Med. 2024 Oct 1;24(4):259-261. doi: 10.4103/tjem.tjem_67_24. eCollection 2024 Oct-Dec. Turk J Emerg Med. 2024. PMID: 39564442 Free PMC article.
-
Structural neural plasticity evoked by rapid-acting antidepressant interventions.Nat Rev Neurosci. 2024 Nov 18. doi: 10.1038/s41583-024-00876-0. Online ahead of print. Nat Rev Neurosci. 2024. PMID: 39558048 Review.
-
Deep phenotypic profiling of neuroactive drugs in larval zebrafish.Nat Commun. 2024 Nov 17;15(1):9955. doi: 10.1038/s41467-024-54375-y. Nat Commun. 2024. PMID: 39551797 Free PMC article.
-
Use of esketamine for tracheoscopic drug injection: a randomized controlled trial.Front Med (Lausanne). 2024 Oct 25;11:1479741. doi: 10.3389/fmed.2024.1479741. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39526245 Free PMC article.
-
Ketamine and chronic treatment-resistant depression: real-world practice and after relapse.BMC Psychiatry. 2024 Oct 28;24(1):745. doi: 10.1186/s12888-024-06203-2. BMC Psychiatry. 2024. PMID: 39468512 Free PMC article.
References
-
- Aalto S, Hirvonen J, Kajander J, Scheinin H, Någren K, Vilkman H, Gustafsson L, Syvälahti E, Hietala J. (2002) Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology (Berl) 164:401–406. - PubMed
-
- Abel KM, Allin MP, Hemsley DR, Geyer MA. (2003) Low dose ketamine increases prepulse inhibition in healthy men. Neuropharmacology 44:729–737. - PubMed
-
- Adamowicz P, Kala M. (2005) Urinary excretion rates of ketamine and norketamine following therapeutic ketamine administration: method and detection window considerations. J Anal Toxicol 29:376–382. - PubMed
-
- Adams JD, Jr, Baillie TA, Trevor AJ, Castagnoli N., Jr (1981) Studies on the biotransformation of ketamine: 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom 8:527–538. - PubMed
-
- Adams JD, Castagnoli N, Trevor AJ. (1978) Quantitative analysis of ketamine enantiomers. Proc West Pharmacol Soc 21:471–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
