Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States
- PMID: 29954737
- PMCID: PMC6202193
- DOI: 10.1161/CIRCULATIONAHA.118.035418
Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States
Erratum in
-
Correction to: Outcomes Associated with Apixaban Use in End-Stage Kidney Disease Patients with Atrial Fibrillation in the United States.Circulation. 2018 Oct 9;138(15):e425. doi: 10.1161/CIR.0000000000000620. Circulation. 2018. PMID: 30354519 No abstract available.
Abstract
Background: Patients with end-stage kidney disease (ESKD) on dialysis were excluded from clinical trials of direct oral anticoagulants for atrial fibrillation (AF). Recent data have raised concerns regarding the safety of dabigatran and rivaroxaban, but apixaban has not been evaluated despite current labeling supporting its use in this population. The goal of this study was to determine patterns of apixaban use and its associated outcomes in dialysis-dependent patients with ESKD and AF.
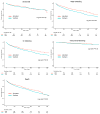
Methods: We performed a retrospective cohort study of Medicare beneficiaries included in the United States Renal Data System (October 2010 to December 2015). Eligible patients were those with ESKD and AF undergoing dialysis who initiated treatment with an oral anticoagulant. Because of the small number of dabigatran and rivaroxaban users, outcomes were only assessed in patients treated with apixaban or warfarin. Apixaban and warfarin patients were matched (1:3) based on prognostic score. Differences between groups in survival free of stroke or systemic embolism, major bleeding, gastrointestinal bleeding, intracranial bleeding, and death were assessed using Kaplan-Meier analyses. Hazard ratios (HRs) and 95% CIs were derived from Cox regression analyses.
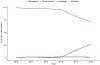
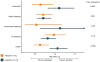
Results: The study population consisted of 25 523 patients (45.7% women; 68.2±11.9 years of age), including 2351 patients on apixaban and 23 172 patients on warfarin. An annual increase in apixaban prescriptions was observed after its marketing approval at the end of 2012, such that 26.6% of new anticoagulant prescriptions in 2015 were for apixaban. In matched cohorts, there was no difference in the risks of stroke/systemic embolism between apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P=0.29), but apixaban was associated with a significantly lower risk of major bleeding (HR, 0.72; 95% CI, 0.59-0.87; P<0.001). In sensitivity analyses, standard-dose apixaban (5 mg twice a day; n=1034) was associated with significantly lower risks of stroke/systemic embolism and death as compared with either reduced-dose apixaban (2.5 mg twice a day; n=1317; HR, 0.61; 95% CI, 0.37-0.98; P=0.04 for stroke/systemic embolism; HR, 0.64; 95% CI, 0.45-0.92; P=0.01 for death) or warfarin (HR, 0.64; 95% CI, 0.42-0.97; P=0.04 for stroke/systemic embolism; HR, 0.63; 95% CI, 0.46-0.85; P=0.003 for death).
Conclusions: Among patients with ESKD and AF on dialysis, apixaban use may be associated with a lower risk of major bleeding compared with warfarin, with a standard 5 mg twice a day dose also associated with reductions in thromboembolic and mortality risk.
Keywords: anticoagulation; atrial fibrillation; bleeding; dialysis; stroke prevention.
Conflict of interest statement
Figures
Comment in
-
Apixaban for End-Stage Kidney Disease.Circulation. 2018 Oct 9;138(15):1534-1536. doi: 10.1161/CIRCULATIONAHA.118.036449. Circulation. 2018. PMID: 30354524 No abstract available.
-
Long-Term Anticoagulation for Patients Receiving Dialysis.Circulation. 2018 Oct 9;138(15):1530-1533. doi: 10.1161/CIRCULATIONAHA.118.037091. Circulation. 2018. PMID: 30354526 No abstract available.
-
Response by Siontis et al to Letter Regarding Article, "Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States".Circulation. 2019 Mar 19;139(12):1563-1564. doi: 10.1161/CIRCULATIONAHA.118.039290. Circulation. 2019. PMID: 30883218 No abstract available.
-
Letter by Cheen Regarding Article, "Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States".Circulation. 2019 Mar 19;139(12):1561-1562. doi: 10.1161/CIRCULATIONAHA.118.038646. Circulation. 2019. PMID: 30883225 No abstract available.
Similar articles
-
Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients With Nonvalvular Atrial Fibrillation.J Am Heart Assoc. 2018 Apr 13;7(8):e008643. doi: 10.1161/JAHA.118.008643. J Am Heart Assoc. 2018. PMID: 29654196 Free PMC article.
-
Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation.J Am Heart Assoc. 2016 Jun 13;5(6):e003725. doi: 10.1161/JAHA.116.003725. J Am Heart Assoc. 2016. PMID: 27412905 Free PMC article.
-
Direct-Acting Oral Anticoagulants Versus Warfarin in Medicare Patients With Chronic Kidney Disease and Atrial Fibrillation.Stroke. 2020 Aug;51(8):2364-2373. doi: 10.1161/STROKEAHA.120.028934. Epub 2020 Jul 9. Stroke. 2020. PMID: 32640949
-
Novel oral anticoagulants for stroke prevention in atrial fibrillation: focus on apixaban.Adv Ther. 2012 Jun;29(6):491-507. doi: 10.1007/s12325-012-0026-8. Epub 2012 Jun 7. Adv Ther. 2012. PMID: 22684583 Review.
-
Factor Xa Inhibitors Versus Vitamin K Antagonists in Atrial Fibrillation Patients with End-Stage Kidney Disease on Dialysis: A Meta-Analysis.Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296241271423. doi: 10.1177/10760296241271423. Clin Appl Thromb Hemost. 2024. PMID: 39140874 Free PMC article. Review.
Cited by
-
Apixaban anti-Xa level monitoring in treatment of acute upper extremity deep vein thrombosis for patient on chronic hemodialysis: a case report.Thromb J. 2021 Apr 1;19(1):23. doi: 10.1186/s12959-021-00277-8. Thromb J. 2021. PMID: 33794913 Free PMC article.
-
Balancing the Risks of Stroke and Bleeding in Patients With Atrial Fibrillation and Renal Dysfunction.JACC Adv. 2024 Mar 8;3(4):100883. doi: 10.1016/j.jacadv.2024.100883. eCollection 2024 Apr. JACC Adv. 2024. PMID: 38939661 Free PMC article.
-
Apixaban Downregulates Endothelial Inflammatory and Prothrombotic Phenotype in an In Vitro Model of Endothelial Dysfunction in Uremia.Cardiovasc Drugs Ther. 2021 Jun;35(3):521-532. doi: 10.1007/s10557-020-07010-z. Cardiovasc Drugs Ther. 2021. PMID: 32651897
-
Risk factors for major bleeding in patients with atrial fibrillation and CKD G3-G5D on oral anticoagulants.Clin Kidney J. 2024 Jul 5;17(8):sfae206. doi: 10.1093/ckj/sfae206. eCollection 2024 Aug. Clin Kidney J. 2024. PMID: 39421237 Free PMC article.
-
Therapeutic Strategies for Disseminated Intravascular Coagulation Associated with Aortic Aneurysm.Int J Mol Sci. 2022 Jan 24;23(3):1296. doi: 10.3390/ijms23031296. Int J Mol Sci. 2022. PMID: 35163216 Free PMC article. Review.
References
-
- Bonde AN, Lip GY, Kamper AL, Hansen PR, Lamberts M, Hommel K, Hansen ML, Gislason GH, Torp-Pedersen C, Olesen JB. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol. 2014;64:2471–2482. - PubMed
-
- Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27:3816–3822. - PubMed
-
- Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Humphries KH, Tu JV, Behlouli H, Guo H, Pilote L. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

