Removal of Mn (II) by Sodium Alginate/Graphene Oxide Composite Double-Network Hydrogel Beads from Aqueous Solutions
- PMID: 30013177
- PMCID: PMC6048064
- DOI: 10.1038/s41598-018-29133-y
Removal of Mn (II) by Sodium Alginate/Graphene Oxide Composite Double-Network Hydrogel Beads from Aqueous Solutions
Abstract
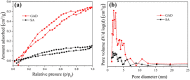
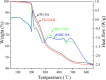
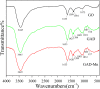
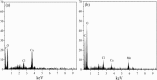
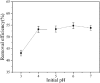
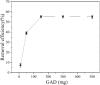
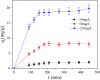
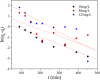
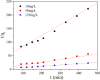
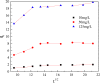
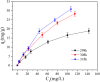
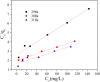
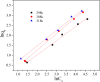
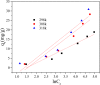
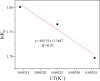
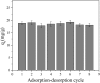
After the successful preparation of empirical double network hydrogel beads from graphene oxide/sodium alginate(GO/SA), its cationic metal adsorption performance in aqueous solutions were investigated. Taking Mn(II) as an example, the contribution of several factors including pH, bead dosage, temperature, contact time and initial concentration ions to adsorption efficiency were examined. The Transmission Electron Microscopy (TEM) results indicate that the GO/SA double (GAD) network hydrogel bead strongly interpenetrate and the adsorption of Mn(II) is mainly influenced by solution pH, bead dose and temperature. The GAD beads exhibit an excellent adsorption capacity of 56.49 mg g-1. The adsorption process fit both Pseudo-second order kinetic model (R2 > 0.97) and the Freundlich adsorption isotherm (R2 > 0.99) and is spontaneous. After seven rounds of adsorption-desorption cycle, the adsorption capacity of GAD hydrogel remained unchanged at 18.11 mg/g.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics.Nanomaterials (Basel). 2021 Feb 25;11(3):568. doi: 10.3390/nano11030568. Nanomaterials (Basel). 2021. PMID: 33668774 Free PMC article.
-
Batch adsorption and desorption studies on the removal of lead (II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads.Int J Biol Macromol. 2017 Nov;104(Pt B):1483-1494. doi: 10.1016/j.ijbiomac.2017.04.120. Epub 2017 May 1. Int J Biol Macromol. 2017. PMID: 28472685
-
Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel.Chemosphere. 2021 May;270:129419. doi: 10.1016/j.chemosphere.2020.129419. Epub 2020 Dec 26. Chemosphere. 2021. PMID: 33418222
-
The impact of functionalized CNT in the network of sodium alginate-based nanocomposite beads on the removal of Co(II) ions from aqueous solutions.J Hazard Mater. 2016 Jul 15;312:224-233. doi: 10.1016/j.jhazmat.2016.03.074. Epub 2016 Mar 26. J Hazard Mater. 2016. PMID: 27037477
-
Removal of Sb(III) by 3D-reduced graphene oxide/sodium alginate double-network composites from an aqueous batch and fixed-bed system.Sci Rep. 2021 Nov 17;11(1):22374. doi: 10.1038/s41598-021-01788-0. Sci Rep. 2021. PMID: 34789761 Free PMC article.
Cited by
-
Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics.Nanomaterials (Basel). 2021 Feb 25;11(3):568. doi: 10.3390/nano11030568. Nanomaterials (Basel). 2021. PMID: 33668774 Free PMC article.
-
Advancing fabrication and properties of three-dimensional graphene-alginate scaffolds for application in neural tissue engineering.RSC Adv. 2019 Nov 12;9(63):36838-36848. doi: 10.1039/c9ra07481c. eCollection 2019 Nov 11. RSC Adv. 2019. PMID: 35539075 Free PMC article.
-
Carbon Nanomaterials for the Treatment of Heavy Metal-Contaminated Water and Environmental Remediation.Nanoscale Res Lett. 2019 Nov 11;14(1):341. doi: 10.1186/s11671-019-3167-8. Nanoscale Res Lett. 2019. PMID: 31712991 Free PMC article. Review.
-
Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel.Nanomaterials (Basel). 2018 Nov 20;8(11):957. doi: 10.3390/nano8110957. Nanomaterials (Basel). 2018. PMID: 30463340 Free PMC article.
-
Processing and modification of hydrogel and its application in emerging contaminant adsorption and in catalyst immobilization: a review.Environ Sci Pollut Res Int. 2020 Apr;27(12):12967-12994. doi: 10.1007/s11356-020-08096-6. Epub 2020 Mar 2. Environ Sci Pollut Res Int. 2020. PMID: 32124301 Review.
References
-
- Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of Environmental Chemical Engineering. 2017;5:2782–2799. doi: 10.1016/j.jece.2017.05.029. - DOI
-
- XU Y, et al. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere. 2017;27:193–204. doi: 10.1016/S1002-0160(17)60310-2. - DOI
-
- Gialamouidis D, Mitrakas M, Liakopoulou-Kyriakides M. Equilibrium, thermodynamic and kinetic studies on biosorption of Mn(II) from aqueous solution by Pseudomonas sp., Staphylococcus xylosus and Blakeslea trispora cells. J. Hazard. Mater. 2010;182:672–680. doi: 10.1016/j.jhazmat.2010.06.084. - DOI - PubMed
-
- Qiu Y-W. Bioaccumulation of heavy metals both in wild and mariculture food chains in Daya Bay, South China. Estuar. Coast. Shelf Sci. 2015;163:7–14. doi: 10.1016/j.ecss.2015.05.036. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

