A comprehensive review on tyrosinase inhibitors
- PMID: 30734608
- PMCID: PMC6327992
- DOI: 10.1080/14756366.2018.1545767
A comprehensive review on tyrosinase inhibitors
Abstract
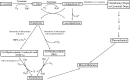
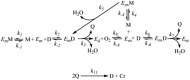
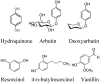
Tyrosinase is a multi-copper enzyme which is widely distributed in different organisms and plays an important role in the melanogenesis and enzymatic browning. Therefore, its inhibitors can be attractive in cosmetics and medicinal industries as depigmentation agents and also in food and agriculture industries as antibrowning compounds. For this purpose, many natural, semi-synthetic and synthetic inhibitors have been developed by different screening methods to date. This review has focused on the tyrosinase inhibitors discovered from all sources and biochemically characterised in the last four decades.
Keywords: Tyrosinase; antibrowning compounds; depigmentation agents; inhibitor.
Figures


Similar articles
-
Flavonoid derivatives as potent tyrosinase inhibitors - a survey of recent findings between 2008-2013.Curr Top Med Chem. 2014;14(12):1486-93. doi: 10.2174/1568026614666140523120741. Curr Top Med Chem. 2014. PMID: 24853561 Review.
-
Inhibitory kinetics of novel 2,3-dihydro-1H-inden-1-one chalcone-like derivatives on mushroom tyrosinase.Bioorg Med Chem Lett. 2015 Dec 1;25(23):5495-9. doi: 10.1016/j.bmcl.2015.10.071. Epub 2015 Oct 24. Bioorg Med Chem Lett. 2015. PMID: 26526213
-
Hydroxyphenyl thiosemicarbazones as inhibitors of mushroom tyrosinase and antibrowning agents.Food Chem. 2020 Jan 15;303:125310. doi: 10.1016/j.foodchem.2019.125310. Epub 2019 Aug 1. Food Chem. 2020. PMID: 31473456
-
Inhibitory Kinetics of Azachalcones and their Oximes on Mushroom Tyrosinase: A Facile Solid-state Synthesis.Chem Biodivers. 2016 May;13(5):531-8. doi: 10.1002/cbdv.201500168. Chem Biodivers. 2016. PMID: 27061023
-
Novel tyrosinase inhibitors from natural resources - their computational studies.Curr Med Chem. 2012;19(14):2262-72. doi: 10.2174/092986712800229041. Curr Med Chem. 2012. PMID: 22414108 Review.
Cited by
-
Tyrosinase Inhibitory Ability and In Vitro, In Vivo Acute Oral and In Silico Toxicity Evaluation of Extracts Obtained from Algerian Fir (Abiesnumidica de Lannoy ex CARRIERE) Needles.Plants (Basel). 2022 Sep 14;11(18):2389. doi: 10.3390/plants11182389. Plants (Basel). 2022. PMID: 36145790 Free PMC article.
-
Hydroxypyrone derivatives in drug discovery: from chelation therapy to rational design of metalloenzyme inhibitors.RSC Med Chem. 2022 Jul 29;13(10):1127-1149. doi: 10.1039/d2md00175f. eCollection 2022 Oct 19. RSC Med Chem. 2022. PMID: 36325396 Free PMC article. Review.
-
Anserine/Carnosine-Rich Extract from Thai Native Chicken Suppresses Melanogenesis via Activation of ERK Signaling Pathway.Molecules. 2022 Nov 2;27(21):7440. doi: 10.3390/molecules27217440. Molecules. 2022. PMID: 36364267 Free PMC article.
-
Biochemical and In Silico Studies on Triazole Derivatives as Tyrosinase Inhibitors: Potential Treatment of Hyperpigmentation Related Skin Disorders.Med Chem. 2024;20(4):397-413. doi: 10.2174/0115734064271581231219111952. Med Chem. 2024. PMID: 38425108
-
Tyrosinase Inhibitory and Antioxidant Activity of Enzymatic Protein Hydrolysate from Jellyfish (Lobonema smithii).Foods. 2022 Feb 21;11(4):615. doi: 10.3390/foods11040615. Foods. 2022. PMID: 35206090 Free PMC article.
References
-
- Dembitsky VM, Kilimnik A. Anti-melanoma agents derived from fungal species. M J Pharma 2016;1:1–16.
-
- Maghsoudi S, Adibi H, Hamzeh M, et al. . Kinetic of mushroom tyrosinase inhibition by benzaldehyde derivatives. J Rep Pharma Sci 2013;2:156–64.
-
- Halaouli S, Asther M, Kruus K, et al. . Characterization of a new tyrosinase from Pycnoporus species with high potential for food technological applications. J Appl Microbiol 2005;98:332–43. - PubMed
-
- Sahu RK, Roy A, Dwivedi J, Jha AK. Promotion and computation of inhibitory effect on tyrosinase activity of herbal cream by incorporating indigenous medicinal plants. Pak J Biol Sci 2014;17:146–50. - PubMed
-
- Jeon SH, Jong-Uk HK, Kwang-Hoon K. Inhibitory effects on L-dopa oxidation of tyrosinase by skin-whitening agents. Bull Korean Chem Soc 2005;26:1135–7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
