Deletion of Osteopontin Enhances β₂-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes
- PMID: 30897705
- PMCID: PMC6470638
- DOI: 10.3390/ijms20061396
Deletion of Osteopontin Enhances β₂-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes
Abstract
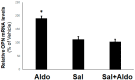
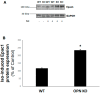
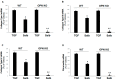
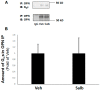
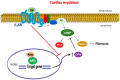
Cardiac β₂-adrenergic receptors (ARs) are known to inhibit collagen production and fibrosis in cardiac fibroblasts and myocytes. The β₂AR is a Gs protein-coupled receptor (GPCR) and, upon its activation, stimulates the generation of cyclic 3',5'-adenosine monophosphate (cAMP). cAMP has two effectors: protein kinase A (PKA) and the exchange protein directly activated by cAMP (Epac). Epac1 has been shown to inhibit cardiac fibroblast activation and fibrosis. Osteopontin (OPN) is a ubiquitous pro-inflammatory cytokine, which also mediates fibrosis in several tissues, including the heart. OPN underlies several cardiovascular pathologies, including atherosclerosis and cardiac adverse remodeling. We found that the cardiotoxic hormone aldosterone transcriptionally upregulates OPN in H9c2 rat cardiac myoblasts-an effect prevented by endogenous β₂AR activation. Additionally, CRISPR-mediated OPN deletion enhanced cAMP generation in response to both β₁AR and β₂AR activation in H9c2 cardiomyocytes, leading to the upregulation of Epac1 protein levels. These effects rendered β₂AR stimulation capable of completely abrogating transforming growth factor (TGF)-β-dependent fibrosis in OPN-lacking H9c2 cardiomyocytes. Finally, OPN interacted constitutively with Gαs subunits in H9c2 cardiac cells. Thus, we uncovered a direct inhibitory role of OPN in cardiac β₂AR anti-fibrotic signaling via cAMP/Epac1. OPN blockade could be of value in the treatment and/or prevention of cardiac fibrosis.
Keywords: CRISPR; Epac1; cAMP; cardiac myocytes; fibrosis; osteopontin; signal transduction; β2-adrenergic receptor.
Conflict of interest statement
The authors declare no conflict of interest related to this publication.
Figures

Similar articles
-
Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):3991-6. doi: 10.1073/pnas.1416163112. Epub 2015 Mar 17. Proc Natl Acad Sci U S A. 2015. PMID: 25829540 Free PMC article.
-
Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy.Cardiovasc Res. 2015 Jan 1;105(1):55-64. doi: 10.1093/cvr/cvu242. Epub 2014 Nov 19. Cardiovasc Res. 2015. PMID: 25411381
-
cAMP-mediated beta-adrenergic signaling negatively regulates Gq-coupled receptor-mediated fetal gene response in cardiomyocytes.J Mol Cell Cardiol. 2008 Dec;45(6):761-9. doi: 10.1016/j.yjmcc.2008.09.120. Epub 2008 Sep 25. J Mol Cell Cardiol. 2008. PMID: 18851973
-
The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology.Cells. 2019 Nov 29;8(12):1543. doi: 10.3390/cells8121543. Cells. 2019. PMID: 31795450 Free PMC article. Review.
-
Compartmentalization of beta-adrenergic signals in cardiomyocytes.Circ Res. 2011 Jul 8;109(2):231-44. doi: 10.1161/CIRCRESAHA.110.231340. Circ Res. 2011. PMID: 21737818 Free PMC article. Review.
Cited by
-
ADRB2 inhibition combined with antioxidant treatment alleviates lung fibrosis by attenuating TGFβ/SMAD signaling in lung fibroblasts.Cell Death Discov. 2023 Nov 4;9(1):407. doi: 10.1038/s41420-023-01702-9. Cell Death Discov. 2023. PMID: 37923730 Free PMC article.
-
The Role of Osteopontin in Tumor Progression Through Tumor-Associated Macrophages.Front Oncol. 2022 Jul 8;12:953283. doi: 10.3389/fonc.2022.953283. eCollection 2022. Front Oncol. 2022. PMID: 35898884 Free PMC article. Review.
-
Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure.Int J Mol Sci. 2024 Jul 28;25(15):8240. doi: 10.3390/ijms25158240. Int J Mol Sci. 2024. PMID: 39125809 Free PMC article.
-
An Overview of the Mechanism behind Excessive Volume of Pericardial Fat in Heart Failure.J Obes Metab Syndr. 2023 Dec 30;32(4):322-329. doi: 10.7570/jomes23042. Epub 2023 Dec 1. J Obes Metab Syndr. 2023. PMID: 38036419 Free PMC article. Review.
-
Cilostazol Attenuates AngII-Induced Cardiac Fibrosis in apoE Deficient Mice.Int J Mol Sci. 2022 Aug 13;23(16):9065. doi: 10.3390/ijms23169065. Int J Mol Sci. 2022. PMID: 36012328 Free PMC article.
References
-
- Desimine V.L., McCrink K.A., Parker B.M., Wertz S.L., Maning J., Lymperopoulos A. Biased Agonism/Antagonism of Cardiovascular GPCRs for Heart Failure Therapy. Int. Rev. Cell. Mol. Biol. 2018;339:41–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

