Elevated level of mitochondrial reactive oxygen species via fatty acid β-oxidation in cancer stem cells promotes cancer metastasis by inducing epithelial-mesenchymal transition
- PMID: 31196164
- PMCID: PMC6567550
- DOI: 10.1186/s13287-019-1265-2
Elevated level of mitochondrial reactive oxygen species via fatty acid β-oxidation in cancer stem cells promotes cancer metastasis by inducing epithelial-mesenchymal transition
Abstract
Background: Cancer stem cells (CSCs) play a critical role in tumor development and progression and are involved in cancer metastasis. The role of reactive oxygen species (ROS) in CSCs and cancer metastasis remains controversial. The aim of the present study was to investigate the correlation between ROS level of CSCs and cancer metastasis and to explore the possible underlying molecular mechanisms.
Methods: Four different cell lines were used to isolate tumor spheres and to analyze intrinsic properties of tumor sphere cells including proliferation, self-renewal potential, differentiation, drug-resistance and cancer metastasis in vitro and in vivo. ROS assays were used to detect the intracellular ROS level of tumor spheres cells. Gene expression analysis and western blot were used to investigate the underlying mechanisms of ROS in regulating cancer metastasis.
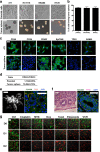
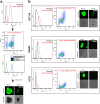
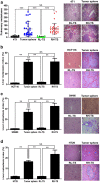
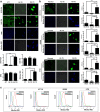
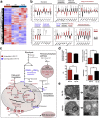
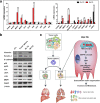
Results: Tumor spheres possessed the characteristic features of CSCs, and ROS-high tumor spheres (RH-TS) displayed elevated mitochondrial ROS level exclusively drove metastasis formation. The gene expression analysis showed elevated fatty acid β-oxidation, downregulation of epithelial marker upregulation of mesenchymal markers, and the activation of MAP kinase cascades. Furthermore, 14 up-regulated genes in RH-TS cells were associated with reduced overall survival of different cancer patients.
Conclusions: Our findings demonstrate that CSCs characterized by elevated mitochondrial ROS level potentiate cancer metastasis. Mechanistically, elevated mitochondrial ROS via fatty acid β-oxidation, activates the MAPK cascades, resulting in the epithelial-mesenchymal transition (EMT) process of RH-TS cells, thereby potentiating caner invasion and metastasis. Therefore, _targeting mitochondrial ROS might provide a promising approach to prevent and alleviate cancer metastasis induced by RH-TS cells.
Keywords: Cancer metastasis; Cancer stem cells; Epithelial-mesenchymal transition; Mitochondria; ROS; Tumor sphere.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling.J Hematol Oncol. 2015 Mar 11;8:23. doi: 10.1186/s13045-015-0119-3. J Hematol Oncol. 2015. PMID: 25879771 Free PMC article.
-
FOXM1-Induced PRX3 Regulates Stemness and Survival of Colon Cancer Cells via Maintenance of Mitochondrial Function.Gastroenterology. 2015 Oct;149(4):1006-16.e9. doi: 10.1053/j.gastro.2015.06.007. Epub 2015 Jun 17. Gastroenterology. 2015. PMID: 26091938
-
MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma.J Exp Clin Cancer Res. 2019 Mar 25;38(1):136. doi: 10.1186/s13046-019-1135-x. J Exp Clin Cancer Res. 2019. PMID: 30909929 Free PMC article.
-
_targeting redox regulation and autophagy systems in cancer stem cells.Clin Exp Med. 2023 Sep;23(5):1405-1423. doi: 10.1007/s10238-022-00955-5. Epub 2022 Dec 6. Clin Exp Med. 2023. PMID: 36473988 Review.
-
Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation.Mol Cancer. 2017 Jan 30;16(1):10. doi: 10.1186/s12943-016-0577-4. Mol Cancer. 2017. PMID: 28137309 Free PMC article. Review.
Cited by
-
Role of anoikis-related gene PLK1 in kidney renal papillary cell carcinoma: a bioinformatics analysis and preliminary verification on promoting proliferation and migration.Front Pharmacol. 2023 Jun 29;14:1211675. doi: 10.3389/fphar.2023.1211675. eCollection 2023. Front Pharmacol. 2023. PMID: 37456749 Free PMC article.
-
The multifaceted role of reactive oxygen species in tumorigenesis.Cell Mol Life Sci. 2020 Nov;77(22):4459-4483. doi: 10.1007/s00018-020-03536-5. Epub 2020 May 1. Cell Mol Life Sci. 2020. PMID: 32358622 Free PMC article. Review.
-
Antioxidant genes in cancer and metabolic diseases: Focusing on Nrf2, Sestrin, and heme oxygenase 1.Int J Biol Sci. 2024 Sep 9;20(12):4888-4907. doi: 10.7150/ijbs.98846. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309448 Free PMC article. Review.
-
_targeting lipid metabolism for ferroptotic cancer therapy.Apoptosis. 2023 Feb;28(1-2):81-107. doi: 10.1007/s10495-022-01795-0. Epub 2022 Nov 18. Apoptosis. 2023. PMID: 36399287 Review.
-
Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation.Cells. 2021 Apr 29;10(5):1056. doi: 10.3390/cells10051056. Cells. 2021. PMID: 33946927 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

