Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis
- PMID: 31290993
- PMCID: PMC6625073
- DOI: 10.1001/jamanetworkopen.2019.6879
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis
Abstract
Importance: The beneficial role of immunotherapy and the clinical relevance of current biomarkers in non-small cell lung cancer (NSCLC) remain inconclusive; thus, appropriate strategies and reliable predictors need further definition.
Objectives: To evaluate the association of clinical outcomes with immune checkpoint inhibitors, tumor vaccines, and cellular immunotherapy in patients with advanced NSCLC and to explore appropriate strategies, candidates, and predictors.
Data sources: The PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases were searched from inception to June 2018, using relevant search keywords and Medical Subject Headings (MeSH) terms, including tumor vaccine, cellular immunotherapy, immune checkpoint inhibitor, cytotoxic T-lymphocyte-associated protein 4, programmed death-ligand 1, programmed death receptor 1, and non-small cell lung carcinoma. Systematic reviews, meta-analyses, references, and conference proceedings were manually searched.
Study selection: English-language randomized clinical trials with available data that measured overall survival (OS), progression-free survival (PFS), or objective response rate comparing immune checkpoint inhibitors, tumor vaccines, or cellular immunotherapy with conventional therapy for patients with advanced or metastatic NSCLC were included. Thirty-one immunotherapy randomized clinical trials were included, and multicohort data included next-generation sequencing data from patients with advanced NCSLC.
Data extraction and synthesis: Hazard ratios and 95% CIs were pooled to estimate the survival increases in OS and PFS. Dichotomous data, such as object response rate data, were analyzed using the risk ratio. Mantel-Haenszel random-effects model was used. I2 was used to assess the heterogeneity between trials; an I2 value exceeding 50% indicated the existence of substantial heterogeneity. Analyses took place from February 1, 2018, to August 31, 2018.
Main outcomes and measures: Primary outcomes were OS and PFS.
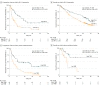
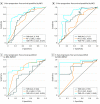
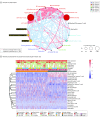
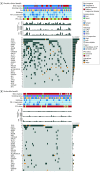
Results: In total, 14 395 patients (9500 [66.0%] men) were included in the meta-analysis, and 1833 patients (mean [SD], 65.2 [9.9] years; 1063 [58.0%] men) were included in the individual patient-level study. Compared with conventional therapy, immunotherapy was associated with significantly longer OS (hazard ratio, 0.76; 95% CI, 0.71-0.82; P < .001) and PFS (hazard ratio, 0.76; 95% CI, 0.70-0.83; P < .001). The best checkpoint blockade strategy was first-line pembrolizumab with platinum-based chemotherapy. The combined predictive utility of programmed cell death ligand 1 (PD-L1) expression and tumor mutation burden (TMB) was associated with predictive prognosis (whole-exome sequencing: 1-year PFS area under the receiver operating characteristic curve [AUC], 0.829; 3-year PFS AUC, 0.839; _targeted next-generation sequencing: 1-year PFS AUC, 0.826; 3-year PFS AUC, 0.948). Moreover, the addition of CD8+ T-cell tumor-infiltrating lymphocytes was associated with improved prognosis predictions for OS (3-year OS AUC, 0.659; 5-year OS AUC, 0.665). RYR1 or MGAM mutations were significantly associated with concomitantly increased durable clinical benefits (RYR1: durable clinical benefit [DCB], 12 of 51 patients [24%]; no durable benefit [NDB], 2 of 55 patients [4%]; P < .001; MGAM: DCB, 12 of 51 patients [24%]; NDB, 0 patients; P < .001), a higher TMB (RYRI: high TMB, 12 of 53 patients [23%]; low TMB, 2 of 53 patients [38%]; P < .001; MGAM: high TMB, 9 of 53 patients [17%]; low TMB, 0 patients; P < .001), and higher PD-L1 expression (RYRI: high PD-L1 expression, 8 of 30 patients [27%]; low PD-L1 expression, 6 of 85 [7.1%]; P < .001; MGAM: high PD-L1 expression, 6 of 30 patients [20%]; low PD-L1 expression, 5 of 85 patients [6%]; P < .001).
Conclusions and relevance: Immunotherapies showed promising clinical outcomes for patients with NSCLC. Pembrolizumab with platinum-based chemotherapy was found to be the most appropriate first-line immune checkpoint inhibitor regimen for advanced NSCLC, and the combined use of PD-L1 expression and TMB was found to be a promising biomarker to evaluate patients' survival and response to precision immunotherapy. The further combination of CD8+ T-cell tumor-infiltrating lymphocytes, PD-L1 expression, and TMB was associated with reliable prognosis. The predictive value of that combination needs to be prospectively validated in large-scale studies.
Conflict of interest statement
Figures

Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3 PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer.Cancer Med. 2021 Apr;10(7):2216-2231. doi: 10.1002/cam4.3649. Epub 2021 Mar 2. Cancer Med. 2021. PMID: 33655698 Free PMC article.
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2017 Dec 16;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Dec 6;12:CD011300. doi: 10.1002/14651858.CD011300.pub3 PMID: 29247502 Free PMC article. Updated. Review.
-
Benefits of combination therapy with immune checkpoint inhibitors and predictive role of tumour mutation burden in hepatocellular carcinoma: A systematic review and meta-analysis.Int Immunopharmacol. 2022 Nov;112:109244. doi: 10.1016/j.intimp.2022.109244. Epub 2022 Sep 18. Int Immunopharmacol. 2022. PMID: 36126410 Review.
Cited by
-
Durable Response to Immunotherapy With Antiangiogenic Drug in Large-Cell Lung Carcinoma With Multiple Fulminant Postoperative Metastases: A Case Report.Front Oncol. 2021 May 20;11:633446. doi: 10.3389/fonc.2021.633446. eCollection 2021. Front Oncol. 2021. PMID: 34094914 Free PMC article.
-
Implication of PD‑L1 polymorphisms rs2297136 on clinical outcomes of patients with advanced NSCLC who received PD‑1 blockades: A retrospective exploratory study.Oncol Lett. 2024 Feb 7;27(4):144. doi: 10.3892/ol.2024.14277. eCollection 2024 Apr. Oncol Lett. 2024. PMID: 38385107 Free PMC article.
-
Cost-Effectiveness Analysis of Sintilimab Plus Chemotherapy in Advanced Non-Squamous Non-Small Cell Lung Cancer: A Societal Perspective.Adv Ther. 2024 Apr;41(4):1436-1449. doi: 10.1007/s12325-024-02808-x. Epub 2024 Feb 15. Adv Ther. 2024. PMID: 38356107
-
Clinical M2 Macrophage-Related Genes Can Serve as a Reliable Predictor of Lung Adenocarcinoma.Front Oncol. 2022 Jul 22;12:919899. doi: 10.3389/fonc.2022.919899. eCollection 2022. Front Oncol. 2022. PMID: 35936688 Free PMC article.
-
Desensitizing Effect of Cancer Cachexia on Immune Checkpoint Inhibitors in Patients With Advanced NSCLC.JTO Clin Res Rep. 2020 Mar 4;1(2):100020. doi: 10.1016/j.jtocrr.2020.100020. eCollection 2020 Jun. JTO Clin Res Rep. 2020. PMID: 34589927 Free PMC article.
References
-
- Horn L, Spigel DR, Vokes EE, et al. . Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933. doi:10.1200/JCO.2017.74.3062 - DOI - PMC - PubMed
-
- Quoix E, Lena H, Losonczy G, et al. . TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212-223. doi:10.1016/S1470-2045(15)00483-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

