Interplay Between Mitochondrial Peroxiredoxins and ROS in Cancer Development and Progression
- PMID: 31500275
- PMCID: PMC6770548
- DOI: 10.3390/ijms20184407
Interplay Between Mitochondrial Peroxiredoxins and ROS in Cancer Development and Progression
Abstract
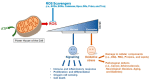
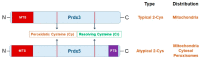
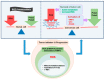
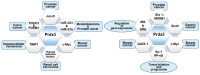
Mitochondria are multifunctional cellular organelles that are major producers of reactive oxygen species (ROS) in eukaryotes; to maintain the redox balance, they are supplemented with different ROS scavengers, including mitochondrial peroxiredoxins (Prdxs). Mitochondrial Prdxs have physiological and pathological significance and are associated with the initiation and progression of various cancer types. In this review, we have focused on signaling involving ROS and mitochondrial Prdxs that is associated with cancer development and progression. An upregulated expression of Prdx3 and Prdx5 has been reported in different cancer types, such as breast, ovarian, endometrial, and lung cancers, as well as in Hodgkin's lymphoma and hepatocellular carcinoma. The expression of Prdx3 and Prdx5 in different types of malignancies involves their association with different factors, such as transcription factors, micro RNAs, tumor suppressors, response elements, and oncogenic genes. The microenvironment of mitochondrial Prdxs plays an important role in cancer development, as cancerous cells are equipped with a high level of antioxidants to overcome excessive ROS production. However, an increased production of Prdx3 and Prdx5 is associated with the development of chemoresistance in certain types of cancers and it leads to further complications in cancer treatment. Understanding the interplay between mitochondrial Prdxs and ROS in carcinogenesis can be useful in the development of anticancer drugs with better proficiency and decreased resistance. However, more _targeted studies are required for exploring the tumor microenvironment in association with mitochondrial Prdxs to improve the existing cancer therapies and drug development.
Keywords: ROS scavengers; mitochondria; peroxiredoxins; reactive oxygen species; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The prognostic values of the peroxiredoxins family in ovarian cancer.Biosci Rep. 2018 Sep 5;38(5):BSR20180667. doi: 10.1042/BSR20180667. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30104402 Free PMC article.
-
Azelaic Acid Exerts Antileukemia Effects against Acute Myeloid Leukemia by Regulating the Prdxs/ROS Signaling Pathway.Oxid Med Cell Longev. 2020 Dec 23;2020:1295984. doi: 10.1155/2020/1295984. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33425206 Free PMC article.
-
New insights into the roles of peroxiredoxins in cancer.Biomed Pharmacother. 2023 Aug;164:114896. doi: 10.1016/j.biopha.2023.114896. Epub 2023 May 19. Biomed Pharmacother. 2023. PMID: 37210897 Review.
-
Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+.Neurosci Lett. 2008 Mar 15;433(3):219-24. doi: 10.1016/j.neulet.2007.12.068. Epub 2008 Jan 17. Neurosci Lett. 2008. PMID: 18262354
-
Peroxiredoxin 1 - an antioxidant enzyme in cancer.J Cell Mol Med. 2017 Jan;21(1):193-202. doi: 10.1111/jcmm.12955. Epub 2016 Sep 21. J Cell Mol Med. 2017. PMID: 27653015 Free PMC article. Review.
Cited by
-
Ethyl β-Carboline-3-Carboxylate Increases Cervical Cancer Cell Apoptosis Through ROS-p38 MAPK Signaling Pathway.In Vivo. 2022 May-Jun;36(3):1178-1187. doi: 10.21873/invivo.12817. In Vivo. 2022. PMID: 35478127 Free PMC article.
-
Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements.Biomolecules. 2019 Nov 13;9(11):735. doi: 10.3390/biom9110735. Biomolecules. 2019. PMID: 31766246 Free PMC article. Review.
-
Prospects of molecular hydrogen in cancer prevention and treatment.J Cancer Res Clin Oncol. 2024 Mar 31;150(4):170. doi: 10.1007/s00432-024-05685-7. J Cancer Res Clin Oncol. 2024. PMID: 38555538 Free PMC article. Review.
-
Assessment of Potential Prognostic Value of Peroxiredoxin 1 in Oral Squamous Cell Carcinoma.Cancer Manag Res. 2021 Jul 15;13:5725-5737. doi: 10.2147/CMAR.S319048. eCollection 2021. Cancer Manag Res. 2021. PMID: 34290530 Free PMC article.
-
A PPIX-binding probe facilitates discovery of PPIX-induced cell death modulation by peroxiredoxin.Commun Biol. 2023 Jun 24;6(1):673. doi: 10.1038/s42003-023-05024-5. Commun Biol. 2023. PMID: 37355765 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

