Recent Progress in Optical Sensors for Biomedical Diagnostics
- PMID: 32235546
- PMCID: PMC7231100
- DOI: 10.3390/mi11040356
Recent Progress in Optical Sensors for Biomedical Diagnostics
Abstract
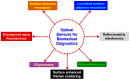
In recent years, several types of optical sensors have been probed for their aptitude in healthcare biosensing, making their applications in biomedical diagnostics a rapidly evolving subject. Optical sensors show versatility amongst different receptor types and even permit the integration of different detection mechanisms. Such conjugated sensing platforms facilitate the exploitation of their neoteric synergistic characteristics for sensor fabrication. This paper covers nearly 250 research articles since 2016 representing the emerging interest in rapid, reproducible and ultrasensitive assays in clinical analysis. Therefore, we present an elaborate review of biomedical diagnostics with the help of optical sensors working on varied principles such as surface plasmon resonance, localised surface plasmon resonance, evanescent wave fluorescence, bioluminescence and several others. These sensors are capable of investigating toxins, proteins, pathogens, disease biomarkers and whole cells in varied sensing media ranging from water to buffer to more complex environments such as serum, blood or urine. Hence, the recent trends discussed in this review hold enormous potential for the widespread use of optical sensors in early-stage disease prediction and point-of-care testing devices.
Keywords: bioluminescence; biomedical diagnostics; biosensors; ellipsometry; evanescent wave; light addressable potentiometric sensors; optical sensors; reflectometric interference spectroscopy; surface enhanced Raman scattering; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Plasmon-enhanced optical sensors: a review.Analyst. 2015 Jan 21;140(2):386-406. doi: 10.1039/c4an01079e. Analyst. 2015. PMID: 25365823 Free PMC article. Review.
-
Nanomaterials for Healthcare Biosensing Applications.Sensors (Basel). 2019 Dec 2;19(23):5311. doi: 10.3390/s19235311. Sensors (Basel). 2019. PMID: 31810313 Free PMC article. Review.
-
Optical biosensors.Essays Biochem. 2016 Jun 30;60(1):91-100. doi: 10.1042/EBC20150010. Essays Biochem. 2016. PMID: 27365039 Free PMC article. Review.
-
Novel Wearable Optical Sensors for Vital Health Monitoring Systems-A Review.Biosensors (Basel). 2023 Jan 23;13(2):181. doi: 10.3390/bios13020181. Biosensors (Basel). 2023. PMID: 36831947 Free PMC article. Review.
-
Research advances on surface plasmon resonance biosensors.Nanoscale. 2022 Jan 20;14(3):564-591. doi: 10.1039/d1nr05400g. Nanoscale. 2022. PMID: 34940766 Review.
Cited by
-
High-Order Multimode Waveguide Interferometer for Optical Biosensing Applications.Sensors (Basel). 2021 May 8;21(9):3254. doi: 10.3390/s21093254. Sensors (Basel). 2021. PMID: 34066692 Free PMC article.
-
Analysis of Polarization Images in the Microphysical Blood Parameters Research for the Hematocrit Diagnostics.Micromachines (Basel). 2022 Dec 16;13(12):2241. doi: 10.3390/mi13122241. Micromachines (Basel). 2022. PMID: 36557540 Free PMC article.
-
Development of a Point-of-Care SPR Sensor for the Diagnosis of Acute Myocardial Infarction.Biosensors (Basel). 2023 Feb 5;13(2):229. doi: 10.3390/bios13020229. Biosensors (Basel). 2023. PMID: 36831995 Free PMC article.
-
Optical biosensors for diagnosis of COVID-19: nanomaterial-enabled particle strategies for post pandemic era.Mikrochim Acta. 2024 May 10;191(6):320. doi: 10.1007/s00604-024-06373-6. Mikrochim Acta. 2024. PMID: 38727849 Free PMC article. Review.
-
Insights into the Formation of DNA-Magnetic Nanoparticle Hybrid Structures: Correlations between Morphological Characterization and Output from Magnetic Biosensor Measurements.ACS Sens. 2020 Nov 25;5(11):3510-3519. doi: 10.1021/acssensors.0c01623. Epub 2020 Nov 3. ACS Sens. 2020. PMID: 33141554 Free PMC article.
References
-
- Altintas Z. In: Biosensors and Nanotechnology—Applications in Health Care Diagnostics. Altintas Z., editor. John Wiley & Sons Press; Hoboken, NJ, USA: 2017.
-
- Altintas Z. Optical Biosensors and Applications to Drug Discovery for Cancer Cases. In: Altintas Z., editor. Biosensors and Nanotechnology: Applications in Health Care Diagnostics. John Wiley & Sons Press; Hoboken, NJ, USA: 2017. pp. 327–348.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

