Dual-Surfactant-Capped Ag Nanoparticles as a Highly Selective and Sensitive Colorimetric Sensor for Citrate Detection
- PMID: 32455188
- PMCID: PMC7240824
- DOI: 10.1021/acsomega.9b04199
Dual-Surfactant-Capped Ag Nanoparticles as a Highly Selective and Sensitive Colorimetric Sensor for Citrate Detection
Erratum in
-
Correction to "Dual-Surfactant-Capped Ag Nanoparticles as a Highly Selective and Sensitive Colorimetric Sensor for Citrate Detection".ACS Omega. 2024 Feb 22;9(9):11025. doi: 10.1021/acsomega.3c09929. eCollection 2024 Mar 5. ACS Omega. 2024. PMID: 38463269 Free PMC article.
Abstract
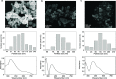
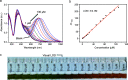
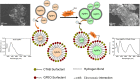
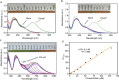
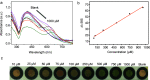
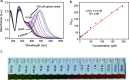
A colorimetric sensor for the detection of citrate ions is reported here using dual-surfactant-capped Ag nanoparticles (dual-AgNP sensor). A mixture of cetyl trimethyl ammonium bromide and a newly prepared gemini nonionic (GFEO) surfactant was used as a capping agent to synthesize dual-surfactant-capped Ag NPs for selective and sensitive citrate detection. The GFEO surfactant was designed with a specific chemical structure to provide strong binding with citrate for selective and sensitive detection. The developed dual-AgNP sensor showed extremely high selectivity toward citrate even in the presence of interfering species. Quantitative detection of citrate was carried out based on the changes in UV-vis absorbance and naked-eye readout. After optimization, the dual-AgNP sensor exhibited a visual detection limit of 25 μM and a low limit of detection of 4.05 nM with a UV-vis spectrometer. The developed citrate sensor performed well with a urine sample, with a high recovery of 99.6%. The prepared solution sensor was constructed on a paper-based analytical device.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Size-tunable Au@Ag nanoparticles for colorimetric and SERS dual-mode sensing of palmatine in traditional Chinese medicine.J Pharm Biomed Anal. 2019 Sep 10;174:123-133. doi: 10.1016/j.jpba.2019.05.045. Epub 2019 May 22. J Pharm Biomed Anal. 2019. PMID: 31163346
-
Rapid visual detection of quaternary ammonium surfactants using citrate-capped silver nanoparticles (Ag NPs) based on hydrophobic effect.Talanta. 2014 Jan;118:90-5. doi: 10.1016/j.talanta.2013.10.009. Epub 2013 Oct 9. Talanta. 2014. PMID: 24274274
-
Correction to "Dual-Surfactant-Capped Ag Nanoparticles as a Highly Selective and Sensitive Colorimetric Sensor for Citrate Detection".ACS Omega. 2024 Feb 22;9(9):11025. doi: 10.1021/acsomega.3c09929. eCollection 2024 Mar 5. ACS Omega. 2024. PMID: 38463269 Free PMC article.
-
Rapid and selective detection of aluminum ion using 1,2,3-triazole-4,5-dicarboxylic acid-functionalized gold nanoparticle-based colorimetric sensor.RSC Adv. 2021 Sep 15;11(49):30635-30645. doi: 10.1039/d1ra04834a. eCollection 2021 Sep 14. RSC Adv. 2021. Retraction in: RSC Adv. 2025 Jan 7;15(1):568. doi: 10.1039/d4ra90132k PMID: 35479866 Free PMC article. Retracted.
-
Polyvinyl alcohol-citrate-stabilized silver nanoparticles as an optical sensor for selective colorimetric determination of sildenafil.Turk J Chem. 2022 Sep 20;46(6):2024-2035. doi: 10.55730/1300-0527.3499. eCollection 2022. Turk J Chem. 2022. PMID: 37621342 Free PMC article.
Cited by
-
Colorimetric Paper-Based Sensors against Cancer Biomarkers.Sensors (Basel). 2022 Apr 22;22(9):3221. doi: 10.3390/s22093221. Sensors (Basel). 2022. PMID: 35590912 Free PMC article. Review.
-
In Situ Functionalization of Silver Nanoparticles by Gallic Acid as a Colorimetric Sensor for Simple Sensitive Determination of Melamine in Milk.ACS Omega. 2021 Aug 27;6(36):23630-23635. doi: 10.1021/acsomega.1c03927. eCollection 2021 Sep 14. ACS Omega. 2021. PMID: 34549161 Free PMC article.
-
Recent Advances in Aptamer Sensors.Sensors (Basel). 2021 Feb 2;21(3):979. doi: 10.3390/s21030979. Sensors (Basel). 2021. PMID: 33540523 Free PMC article. Review.
-
ECL sensor for selective determination of citrate ions as a prostate cancer biomarker using polymer of intrinsic microporosity-1 nanoparticles/nitrogen-doped carbon quantum dots.Anal Bioanal Chem. 2023 Jun;415(14):2727-2736. doi: 10.1007/s00216-023-04672-0. Epub 2023 Apr 12. Anal Bioanal Chem. 2023. PMID: 37042993
-
Dual colorimetric sensing of ascorbic acid and thyroxine using Ag-EGCG-CTAB via a DFT approach.RSC Adv. 2021 Nov 16;11(58):36698-36706. doi: 10.1039/d1ra04204a. eCollection 2021 Nov 10. RSC Adv. 2021. PMID: 35494345 Free PMC article.
References
-
- Liu Z.-H.; Devaraj S.; Yang C.-R.; Yen Y.-P. A new selective chromogenic and fluorogenic sensor for citrate ion. Sens. Actuators, B 2012, 174, 555–562. 10.1016/j.snb.2012.07.030. - DOI
LinkOut - more resources
Full Text Sources

