The adherence-associated Fdp fasciclin I domain protein of the biohydrogen producer Rhodobacter sphaeroides is regulated by the global Prr pathway
- PMID: 33093750
- PMCID: PMC7561615
- DOI: 10.1016/j.ijhydene.2020.07.108
The adherence-associated Fdp fasciclin I domain protein of the biohydrogen producer Rhodobacter sphaeroides is regulated by the global Prr pathway
Abstract
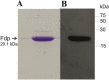
Expression of fdp, encoding a fasciclin I domain protein important for adherence in the hydrogen-producing bacterium Rhodobacter sphaeroides, was investigated under a range of conditions to gain insights into optimization of adherence for immobilization strategies suitable for H2 production. The fdp promoter was linked to a lacZ reporter and expressed in wild type and in PRRB and PRRA mutant strains of the Prr regulatory pathway. Expression was significantly negatively regulated by Prr under all conditions of aerobiosis tested including anaerobic conditions (required for H2 production), and aerobically regardless of growth phase, growth medium complexity or composition, carbon source, heat and cold shock and dark/light conditions. Negative fdp regulation by Prr was reflected in cellular levels of translated Fdp protein. Since Prr is required directly for nitrogenase expression, we propose optimization of Fdp-based adherence in R. sphaeroides for immobilized biohydrogen production by inactivation of the PrrA binding site(s) upstream of fdp.
Keywords: Biohydrogen; Fasciclin domain; Gene regulation; Green energy; Prr two-component system; Rhodobacter sphaeroides.
Crown Copyright © 2020 Published by Elsevier Ltd on behalf of Hydrogen Energy Publications LLC.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1.J Bacteriol. 1998 Aug;180(16):4044-50. doi: 10.1128/JB.180.16.4044-4050.1998. J Bacteriol. 1998. PMID: 9696749 Free PMC article.
-
Introduction of Glyoxylate Bypass Increases Hydrogen Gas Yield from Acetate and l-Glutamate in Rhodobacter sphaeroides.Appl Environ Microbiol. 2019 Jan 9;85(2):e01873-18. doi: 10.1128/AEM.01873-18. Print 2019 Jan 15. Appl Environ Microbiol. 2019. PMID: 30413472 Free PMC article.
-
Complex regulatory activities associated with the histidine kinase PrrB in expression of photosynthesis genes in Rhodobacter sphaeroides 2.4.1.J Bacteriol. 1996 Dec;178(24):7037-46. doi: 10.1128/jb.178.24.7037-7046.1996. J Bacteriol. 1996. PMID: 8955382 Free PMC article.
-
Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase.J Bacteriol. 1995 May;177(10):2695-706. doi: 10.1128/jb.177.10.2695-2706.1995. J Bacteriol. 1995. PMID: 7751278 Free PMC article.
-
In vitro and in vivo analysis of the role of PrrA in Rhodobacter sphaeroides 2.4.1 hemA gene expression.J Bacteriol. 2006 May;188(9):3208-18. doi: 10.1128/JB.188.9.3208-3218.2006. J Bacteriol. 2006. PMID: 16621813 Free PMC article.
Cited by
-
Sticky decisions: The multilayered regulation of adhesin production by bacteria.PLoS Genet. 2023 Mar 2;19(3):e1010648. doi: 10.1371/journal.pgen.1010648. eCollection 2023 Mar. PLoS Genet. 2023. PMID: 36862629 Free PMC article. No abstract available.
-
Membrane Sensor Histidine Kinases: Insights from Structural, Ligand and Inhibitor Studies of Full-Length Proteins and Signalling Domains for Antibiotic Discovery.Molecules. 2021 Aug 23;26(16):5110. doi: 10.3390/molecules26165110. Molecules. 2021. PMID: 34443697 Free PMC article. Review.
References
-
- Basak N., Kumar Jana A., Das D., Saikia D. Photofermentative molecular biohydrogen production by purple non-sulfur (PNS) bacteria in various modes: the present progress and future perspective. Int J Hydrogen Energy. 2014;39:6853–6871.
-
- Wang Y., Zhou P., Gao R. Advances in the genetic modification of Rhodobacter sphaeroides to improve hydrogen production. Renew Sustain Energy Rev. 2016;60:1312–1318.
-
- Ghosh S., Kulsoom Dairkee U., Chowdhury R., Bhattacharya P. Hydrogen from food processing wastes via photofermentation using purple non-sulfur bacteria (PNSB) – a review. Energy Convers Manag. 2017;141:299–314.
-
- Trchounian K., Sawers R.G., Trchounian A. Improving biohydrogen productivity by microbial dark- and photo-fermentations: novel data and future approaches. Renew Sustain Energy Rev. 2017;80:1201–1216.
-
- Oncel S., Sabankay M. Microalgal biohydrogen production considering light energy and mixing time as the two key features for scale-up. Bioresour Technol. 2012;121:228–234. - PubMed
LinkOut - more resources
Full Text Sources
