Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent
- PMID: 9456319
- PMCID: PMC2140175
- DOI: 10.1083/jcb.140.3.591
Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent
Abstract
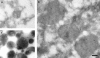
Using a cell-free content mixing assay containing rat liver endosomes and lysosomes in the presence of pig brain cytosol, we demonstrated that after incubation at 37 degrees C, late endosome-lysosome hybrid organelles were formed, which could be isolated by density gradient centrifugation. ImmunoEM showed that the hybrids contained both an endocytosed marker and a lysosomal enzyme. Formation of the hybrid organelles appeared not to require vesicular transport between late endosomes and lysosomes but occurred as a result of direct fusion. Hybrid organelles with similar properties were isolated directly from rat liver homogenates and thus were not an artifact of cell-free incubations. Direct fusion between late endosomes and lysosomes was an N-ethylmaleimide-sensitive factor-dependent event and was inhibited by GDP-dissociation inhibitor, indicating a requirement for a rab protein. We suggest that in cells, delivery of endocytosed ligands to an organelle where proteolytic digestion occurs is mediated by direct fusion of late endosomes with lysosomes. The consequences of this fusion to the maintenance and function of lysosomes are discussed.
Figures
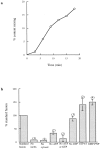
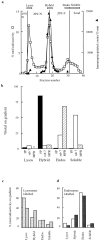
 ) and after (
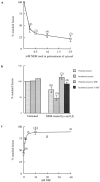
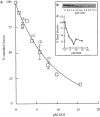
) and after ( ) the incubation of the content mixing assay (lysosomes loaded with 125I-bpIgA). (d) Distribution of total radioactivity before (□) and after (▪) incubation of 125I-Av-ASF–loaded late endosomes with unlabeled lysosomes under the conditions of the content mixing assay. Lysos, lysosomes; Endos, endosomes; N, Nycodenz; F, Ficoll.
) the incubation of the content mixing assay (lysosomes loaded with 125I-bpIgA). (d) Distribution of total radioactivity before (□) and after (▪) incubation of 125I-Av-ASF–loaded late endosomes with unlabeled lysosomes under the conditions of the content mixing assay. Lysos, lysosomes; Endos, endosomes; N, Nycodenz; F, Ficoll.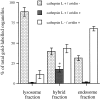

Similar articles
-
The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles.J Cell Biol. 2000 May 29;149(5):1053-62. doi: 10.1083/jcb.149.5.1053. J Cell Biol. 2000. PMID: 10831609 Free PMC article.
-
Live Salmonella recruits N-ethylmaleimide-sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome.J Cell Biol. 2000 Feb 21;148(4):741-53. doi: 10.1083/jcb.148.4.741. J Cell Biol. 2000. PMID: 10684255 Free PMC article.
-
Relationship between endosomes and lysosomes.Biochem Soc Trans. 2001 Aug;29(Pt 4):476-80. doi: 10.1042/bst0290476. Biochem Soc Trans. 2001. PMID: 11498012 Review.
-
Reconstitution of phagosome-lysosome fusion in streptolysin O-permeabilized cells.J Biol Chem. 1997 Jun 27;272(26):16147-51. doi: 10.1074/jbc.272.26.16147. J Biol Chem. 1997. PMID: 9195911
-
Regulation of endosome fusion.Mol Membr Biol. 1999 Jan-Mar;16(1):73-9. doi: 10.1080/096876899294788. Mol Membr Biol. 1999. PMID: 10332740 Review.
Cited by
-
The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles.Mol Biol Cell. 2003 Oct;14(10):4015-27. doi: 10.1091/mbc.e03-01-0040. Epub 2003 Jul 11. Mol Biol Cell. 2003. PMID: 14517315 Free PMC article.
-
Cellular endocytosis and gene delivery.Mol Med. 2010 May-Jun;16(5-6):222-9. doi: 10.2119/molmed.2009.00101. Epub 2010 Feb 3. Mol Med. 2010. PMID: 20454523 Free PMC article. Review.
-
EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation.EMBO J. 2006 Jan 11;25(1):1-12. doi: 10.1038/sj.emboj.7600759. Epub 2005 Jul 28. EMBO J. 2006. PMID: 16052208 Free PMC article.
-
Late endosome motility depends on lipids via the small GTPase Rab7.EMBO J. 2002 Mar 15;21(6):1289-300. doi: 10.1093/emboj/21.6.1289. EMBO J. 2002. PMID: 11889035 Free PMC article.
-
Sequential actions of phosphatidylinositol phosphates regulate phagosome-lysosome fusion.Mol Biol Cell. 2018 Feb 15;29(4):452-465. doi: 10.1091/mbc.E17-07-0464. Epub 2017 Dec 13. Mol Biol Cell. 2018. PMID: 29237821 Free PMC article.
References
-
- Acharya U, Jacobs R, Peters J, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
-
- Blommaart EF, Krause U, Schellens JPM, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. - PubMed

