SMAD3/4-dependent transcriptional activation of the human type VII collagen gene (COL7A1) promoter by transforming growth factor beta
- PMID: 9843964
- PMCID: PMC24524
- DOI: 10.1073/pnas.95.25.14769
SMAD3/4-dependent transcriptional activation of the human type VII collagen gene (COL7A1) promoter by transforming growth factor beta
Abstract
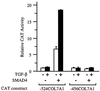
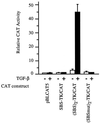
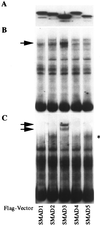
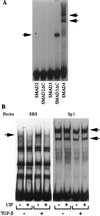
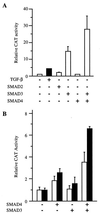
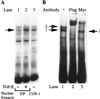
The human type VII collagen gene (COL7A1) recently has been identified as an immediate-early response gene for transforming growth factor beta (TGF-beta)/SMAD signaling pathway. In this study, by using MDA-MB-468 SMAD4-/- breast carcinoma cells, we demonstrate that expression of SMAD4 is an absolute requirement for SMAD-mediated promoter activity. We also demonstrate that the SMAD binding sequence (SBS) representing the TGF-beta response element in the region -496/-444 of the COL7A1 promoter functions as an enhancer in the context of a heterologous promoter. Electrophoretic mobility-shift assays with nuclear extracts from COS-1 cells transfected with expression vectors for SMADs 1-5 indicate that SMAD3 forms a complex with a migration similar to that of the endogenous TGF-beta-specific complex observed in fibroblast extracts. Electrophoretic mobility-shift assays using recombinant glutathione S-transferase-SMAD fusion proteins indicate that both SMAD4 and C-terminally truncated SMAD3, but not SMAD2, can bind the COL7A1 SBS. Coexpression of SMAD3 and SMAD4 in COS-1 cells leads to the formation of two complexes: a DNA/protein complex containing SMAD3 alone and another slower-migrating complex containing both SMAD3 and SMAD4, the latter complex not being detected in fibroblasts. Maximal transactivation of COL7A1 SBS-driven promoters in either MDA-MB-468 carcinoma cells or fibroblasts requires concomitant overexpression of SMAD3 and SMAD4. These data may represent the first identification of a functional homomeric SMAD3 complex regulating a human gene.
Figures
Similar articles
-
Interaction of smad3 with a proximal smad-binding element of the human alpha2(I) procollagen gene promoter required for transcriptional activation by TGF-beta.J Cell Physiol. 2000 Jun;183(3):381-92. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. J Cell Physiol. 2000. PMID: 10797313
-
The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells.Kidney Int. 1999 Oct;56(4):1354-65. doi: 10.1046/j.1523-1755.1999.00680.x. Kidney Int. 1999. PMID: 10504488
-
Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators.Oncogene. 2000 Jul 20;19(31):3546-55. doi: 10.1038/sj.onc.1203693. Oncogene. 2000. PMID: 10918613
-
Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription.J Biol Chem. 2000 Dec 15;275(50):39237-45. doi: 10.1074/jbc.M003339200. J Biol Chem. 2000. PMID: 11007770
-
Tumor suppressor Smad4 is a transforming growth factor beta-inducible DNA binding protein.Mol Cell Biol. 1997 Dec;17(12):7019-28. doi: 10.1128/MCB.17.12.7019. Mol Cell Biol. 1997. PMID: 9372933 Free PMC article.
Cited by
-
Diverse roles of TGF-β/Smads in renal fibrosis and inflammation.Int J Biol Sci. 2011;7(7):1056-67. doi: 10.7150/ijbs.7.1056. Epub 2011 Sep 2. Int J Biol Sci. 2011. PMID: 21927575 Free PMC article. Review.
-
Brd4 inhibition attenuates unilateral ureteral obstruction-induced fibrosis by blocking TGF-β-mediated Nox4 expression.Redox Biol. 2017 Apr;11:390-402. doi: 10.1016/j.redox.2016.12.031. Epub 2016 Dec 30. Redox Biol. 2017. PMID: 28063381 Free PMC article.
-
Pregnancy-specific glycoprotein 9 acts as both a transcriptional _target and a regulator of the canonical TGF-β/Smad signaling to drive breast cancer progression.Clin Transl Med. 2020 Dec;10(8):e245. doi: 10.1002/ctm2.245. Clin Transl Med. 2020. PMID: 33377651 Free PMC article.
-
Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappaB site.Biochem J. 2004 Jul 15;381(Pt 2):413-22. doi: 10.1042/BJ20040058. Biochem J. 2004. PMID: 15086314 Free PMC article.
-
Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome.Int J Hematol. 2002 Apr;75(3):289-97. doi: 10.1007/BF02982044. Int J Hematol. 2002. PMID: 11999358
References
-
- Massagué J, Hata A, Liu F. Trends Cell Biol. 1997;7:187–192. - PubMed
-
- Heldin C H, Miyazono K, ten Dijke P. Nature (London) 1997;390:465–471. - PubMed
-
- Macias-Silva M, Abdollah S, Hoodless P A, Pirone R, Attisano L, Wrana J L. Cell. 1996;87:1215–1224. - PubMed
-
- Zhang Y, Feng X-H, Wu R-Y, Derynck R. Nature (London) 1996;383:168–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

