Liu Jun Zi Tang-A Potential, Multi-Herbal Complementary Therapy for Chemotherapy-Induced Neurotoxicity
- PMID: 29690597
- PMCID: PMC5979528
- DOI: 10.3390/ijms19041258
Liu Jun Zi Tang-A Potential, Multi-Herbal Complementary Therapy for Chemotherapy-Induced Neurotoxicity
Abstract
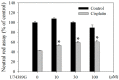
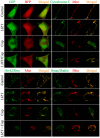
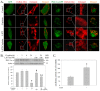
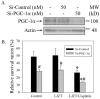
Liu Jun Zi Tang (LJZT) has been used to treat functional dyspepsia and depression, suggesting its effects on gastrointestinal and neurological functions. LJZT is currently used as a complementary therapy to attenuate cisplatin-induced side effects, such as dyspepsia. However, its effect on chemotherapy-induced neuropathic pain or neurotoxicity has rarely been studied. Thus, we explored potential mechanisms underlying LJZT protection against cisplatin-induced neurotoxicity. We observed that LJZT attenuated cisplatin-induced thermal hyperalgesia in mice and apoptosis in human neuroblastoma SH-SY5Y cells. Furthermore, it also attenuated cisplatin-induced cytosolic and mitochondrial free radical formation, reversed the cisplatin-induced decrease in mitochondrial membrane potential, and increased the release of mitochondrial pro-apoptotic factors. LJZT not only activated the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) promoter region, but also attenuated the cisplatin-induced reduction of PGC-1α expression. Silencing of the PGC-1α gene counteracted the protection of LJZT. Taken together, LJZT mediated, through anti-oxidative effect and mitochondrial function regulation, to prevent cisplatin-induced neurotoxicity.
Keywords: Liu Jun Zi Tang; PGC-1α; cisplatin; mitochondria; neurotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Chinese herbal medicine liu jun zi tang and xiang sha liu jun zi tang for functional dyspepsia: meta-analysis of randomized controlled trials.Evid Based Complement Alternat Med. 2012;2012:936459. doi: 10.1155/2012/936459. Epub 2012 Dec 12. Evid Based Complement Alternat Med. 2012. PMID: 23304226 Free PMC article.
-
Overexpression of PGC-1α Influences Mitochondrial Signal Transduction of Dopaminergic Neurons.Mol Neurobiol. 2016 Aug;53(6):3756-3770. doi: 10.1007/s12035-015-9299-7. Epub 2015 Jul 4. Mol Neurobiol. 2016. PMID: 26141122
-
Shaoyao Gancao Tang (SG-Tang), a formulated Chinese medicine, reduces aggregation and exerts neuroprotection in spinocerebellar ataxia type 17 (SCA17) cell and mouse models.Aging (Albany NY). 2019 Feb 13;11(3):986-1007. doi: 10.18632/aging.101804. Aging (Albany NY). 2019. PMID: 30760647 Free PMC article.
-
Chikusetsu saponin V attenuates H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells through Sirt1/PGC-1α/Mn-SOD signaling pathways.Can J Physiol Pharmacol. 2016 Sep;94(9):919-28. doi: 10.1139/cjpp-2015-0262. Epub 2016 Apr 19. Can J Physiol Pharmacol. 2016. PMID: 27332950
-
Dysfunction of the PGC-1α-mitochondria axis confers adriamycin-induced podocyte injury.Am J Physiol Renal Physiol. 2014 Jun 15;306(12):F1410-7. doi: 10.1152/ajprenal.00622.2013. Epub 2014 May 7. Am J Physiol Renal Physiol. 2014. PMID: 24808537
Cited by
-
Effects of alkaloids on peripheral neuropathic pain: a review.Chin Med. 2020 Oct 2;15:106. doi: 10.1186/s13020-020-00387-x. eCollection 2020. Chin Med. 2020. PMID: 33024448 Free PMC article. Review.
-
Therapeutics for Chemotherapy-Induced Peripheral Neuropathy: Approaches with Natural Compounds from Traditional Eastern Medicine.Pharmaceutics. 2022 Jul 5;14(7):1407. doi: 10.3390/pharmaceutics14071407. Pharmaceutics. 2022. PMID: 35890302 Free PMC article. Review.
-
The Effect of Resveratrol or Curcumin on Head and Neck Cancer Cells Sensitivity to the Cytotoxic Effects of Cisplatin.Nutrients. 2020 Aug 26;12(9):2596. doi: 10.3390/nu12092596. Nutrients. 2020. PMID: 32859062 Free PMC article.
-
What Makes the Gut-Lung Axis Working? From the Perspective of Microbiota and Traditional Chinese Medicine.Can J Infect Dis Med Microbiol. 2024 Jan 18;2024:8640014. doi: 10.1155/2024/8640014. eCollection 2024. Can J Infect Dis Med Microbiol. 2024. PMID: 38274122 Free PMC article. Review.
-
Protective Effects of ACY-1215 Against Chemotherapy-Related Cognitive Impairment and Brain Damage in Mice.Neurochem Res. 2019 Nov;44(11):2460-2469. doi: 10.1007/s11064-019-02882-6. Epub 2019 Sep 30. Neurochem Res. 2019. PMID: 31571096
References
-
- Yang J., Huang H., Zhu L.J. World Century Compendium to TCM. Volume 5 World Centry Publishing Corporation; Hackensack, NJ, USA: 2013. Introduction to Formulae of Traditional Chinese Medicine.
-
- Xiao Y., Liu Y.Y., Yu K.Q., Ouyang M.Z., Luo R., Zhao X.S. Chinese herbal medicine liu jun zi tang and xiang sha liu jun zi tang for functional dyspepsia: Meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2012;2012:936459. doi: 10.1155/2012/936459. - DOI - PMC - PubMed
-
- Chao T.H., Fu P.K., Chang C.H., Chang S.N., Chiahung Mao F., Lin C.H., Evidence-based Chinese Medicine Research Group Prescription patterns of Chinese herbal products for post-surgery colon cancer patients in Taiwan. J. Ethnopharmacol. 2014;155:702–708. doi: 10.1016/j.jep.2014.06.012. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

