Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage
- PMID: 32604971
- PMCID: PMC7353213
- DOI: 10.3390/nu12061901
Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage
Abstract
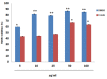
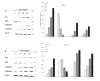
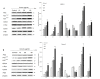
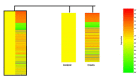
Pancreatic cancer is one of the fatal causes of global cancer-related deaths. Although surgery and chemotherapy are standard treatment options, post-treatment outcomes often end in a poor prognosis. In the present study, we investigated anti-pancreatic cancer and amelioration of radiation-induced oxidative damage by crocin. Crocin is a carotenoid isolated from the dietary herb saffron, a prospect for novel leads as an anti-cancer agent. Crocin significantly reduced cell viability of BXPC3 and Capan-2 by triggering caspase signaling via the downregulation of Bcl-2. It modulated the expression of cell cycle signaling proteins P53, P21, P27, CDK2, c-MYC, Cyt-c and P38. Concomitantly, crocin treatment-induced apoptosis by inducing the release of cytochrome c from mitochondria to cytosol. Microarray analysis of the expression signature of genes induced by crocin showed a substantial number of genes involved in cell signaling pathways and checkpoints (723) are significantly affected by crocin. In mice bearing pancreatic tumors, crocin significantly reduced tumor burden without a change in body weight. Additionally, it showed significant protection against radiation-induced hepatic oxidative damage, reduced the levels of hepatic toxicity and preserved liver morphology. These findings indicate that crocin has a potential role in the treatment, prevention and management of pancreatic cancer.
Keywords: apoptosis; cell cycle; crocin; hepatic injury; pancreatic cancer; radiation.
Conflict of interest statement
Authors declare that there are no conflicts of interest.
Figures


Similar articles
-
DNA fragmentation and cell cycle arrest: a hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line.Asian Pac J Cancer Prev. 2010;11(3):675-9. Asian Pac J Cancer Prev. 2010. PMID: 21039035
-
Chemo-preventive effect of crocin against experimentally-induced hepatocarcinogenesis via regulation of apoptotic and Nrf2 signaling pathways.Environ Toxicol Pharmacol. 2020 Nov;80:103494. doi: 10.1016/j.etap.2020.103494. Epub 2020 Sep 14. Environ Toxicol Pharmacol. 2020. PMID: 32942000
-
Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure.Chem Biol Interact. 2013 May 25;203(3):547-55. doi: 10.1016/j.cbi.2013.03.010. Epub 2013 Mar 21. Chem Biol Interact. 2013. PMID: 23523949
-
Crocetin and Crocin from Saffron in Cancer Chemotherapy and Chemoprevention.Anticancer Agents Med Chem. 2019;19(1):38-47. doi: 10.2174/1871520619666181231112453. Anticancer Agents Med Chem. 2019. PMID: 30599111 Review.
-
A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin.J Pharm Pharmacol. 2017 Nov;69(11):1419-1427. doi: 10.1111/jphp.12776. Epub 2017 Jul 3. J Pharm Pharmacol. 2017. PMID: 28675431 Review.
Cited by
-
Potential therapeutic effects of crocin.Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct;397(10):7395-7420. doi: 10.1007/s00210-024-03131-6. Epub 2024 May 17. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38758225 Review.
-
The oxidant-antioxidant imbalance was involved in the pathogenesis of chronic rhinosinusitis with nasal polyps.Front Immunol. 2024 May 2;15:1380846. doi: 10.3389/fimmu.2024.1380846. eCollection 2024. Front Immunol. 2024. PMID: 38756779 Free PMC article.
-
Carotenoids in Health as Studied by Omics-Related Endpoints.Adv Nutr. 2023 Nov;14(6):1538-1578. doi: 10.1016/j.advnut.2023.09.002. Epub 2023 Sep 9. Adv Nutr. 2023. PMID: 37678712 Free PMC article. Review.
-
Advances on the anti-tumor mechanisms of the carotenoid Crocin.PeerJ. 2023 Jun 29;11:e15535. doi: 10.7717/peerj.15535. eCollection 2023. PeerJ. 2023. PMID: 37404473 Free PMC article. Review.
-
Current Status and De Novo Synthesis of Anti-Tumor Alkaloids in Nicotiana.Metabolites. 2023 Apr 30;13(5):623. doi: 10.3390/metabo13050623. Metabolites. 2023. PMID: 37233664 Free PMC article. Review.
References
-
- National Cancer Institute. M. SEER Cancer Statistics Factsheets. Pancreas Cancer (National Cancer Institute); Bethesda, MD, USA: 2012.
-
- Sohal D.P.S., Kennedy E.B., Khorana A., Copur M.S., Crane C.H., Garrido-Laguna I., Krishnamurthi S., Moravek C., O’Reilly E.M., Philip P.A., et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:2545–2556. doi: 10.1200/JCO.2018.78.9636. - DOI - PMC - PubMed
-
- Groot V.P., Gemenetzis G., Blair A.B., Rivero-Soto R.J., Yu J., Javed A.A., Burkhart R.A., Rinkes I.H.M.B., Molenaar I.Q., Cameron J.L., et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019;269:1154–1162. doi: 10.1097/SLA.0000000000002734. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

