Enhanced Sensitivity and Accuracy of Tb3+-Functionalized Zirconium-Based Bimetallic MOF for Visual Detection of Malachite Green in Fish
- PMID: 39272620
- PMCID: PMC11395321
- DOI: 10.3390/foods13172855
Enhanced Sensitivity and Accuracy of Tb3+-Functionalized Zirconium-Based Bimetallic MOF for Visual Detection of Malachite Green in Fish
Abstract
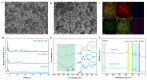
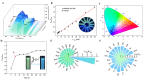
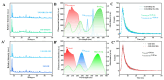
The ratiometric fluorescent probe UiO-OH@Tb, a zirconium-based MOF functionalized with Tb3+, was synthesized using a hydrothermal method. This probe employs the fluorescence resonance energy transfer (FRET) mechanism between Tb3+ and malachite green (MG) for the double-inverse signal ratiometric fluorescence detection of MG. The probe's color shifts from lime green to blue with an increasing concentration of MG. In contrast, the monometallic MOFs' (UiO-OH) probe shows only blue fluorescence quenching due to the inner filter effect (IFE) after interacting with MG. Additionally, the composite fluorescent probe (UiO-OH@Tb) exhibits superior sensitivity, with a detection limit (LOD) of 0.19 μM, which is significantly lower than that of the monometallic MOFs (25 μM). Moreover, the content of MG can be detected on-site (LOD = 0.94 μM) using the RGB function of smartphones. Hence, the UiO-OH@Tb probe is proven to be an ideal material for MG detection, demonstrating significant practical value in real-world applications.
Keywords: FRET; UiO-OH@Tb; ratiometric fluorescence; smartphone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Smartphone-based dual inverse signal MOFs fluorescence sensing for intelligent on-site visual detection of malachite green.Talanta. 2024 Jul 1;274:126039. doi: 10.1016/j.talanta.2024.126039. Epub 2024 Apr 4. Talanta. 2024. PMID: 38604043
-
A ratiometric fluorescent probe for determination of the anthrax biomarker 2,6-pyridinedicarboxylic acid based on a terbium(III)- functionalized UIO-67 metal-organic framework.Mikrochim Acta. 2020 Jan 13;187(2):122. doi: 10.1007/s00604-020-4113-2. Mikrochim Acta. 2020. PMID: 31932902
-
Design of a dual-mode ratiometric fluorescent probe via MOF-on-MOF strategy for Al (III) and pH detection.Anal Chim Acta. 2024 Apr 15;1298:342403. doi: 10.1016/j.aca.2024.342403. Epub 2024 Feb 24. Anal Chim Acta. 2024. PMID: 38462341
-
UiO-66-NH2 MOF-based ratiometric fluorescent probe for the detection of dopamine and reduced glutathione.Talanta. 2020 Dec 1;220:121352. doi: 10.1016/j.talanta.2020.121352. Epub 2020 Jul 6. Talanta. 2020. PMID: 32928390
-
A portable smartphone-assisted Tb-MOF-based agar-slice probe for the rapid and on-site fluorescence assay of malachite green in aquatic products.Food Chem. 2024 Mar 30;437(Pt 1):137883. doi: 10.1016/j.foodchem.2023.137883. Epub 2023 Oct 29. Food Chem. 2024. PMID: 37918152
References
-
- Hashemi S.H., Kaykhaii M., Keikha A.J., Mirmoradzehi E., Sargazi G. Application of response surface methodology for optimization of metal-organic framework based pipette-tip solid phase extraction of organic dyes from seawater and their determination with HPLC. BMC Chem. 2019;13:59. doi: 10.1186/s13065-019-0572-0. - DOI - PMC - PubMed
-
- Ekmen E., Bilici M., Turan E., Tamer U., Zengin A. Surface molecularly-imprinted magnetic nanoparticles coupled with SERS sensing platform for selective detection of malachite green. Sens. Actuators B-Chem. 2020;325:128787. doi: 10.1016/j.snb.2020.128787. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

