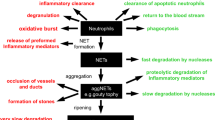
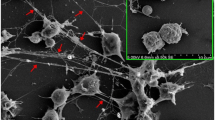
Abstract
Neutrophil NETosis is an important element of host defense as it catapults chromatin out of the cell to trap bacteria, which then are killed, e.g., by the chromatin’s histone component. Also, during sterile inflammation TNF-alpha and other mediators trigger NETosis, which elicits cytotoxic effects on host cells. The same mechanism should apply to other forms of regulated necrosis including pyroptosis, necroptosis, ferroptosis, and cyclophilin D-mediated regulated necrosis. Beyond these toxic effects, extracellular histones also trigger thrombus formation and innate immunity by activating Toll-like receptors and the NLRP3 inflammasome. Thereby, extracellular histones contribute to the microvascular complications of sepsis, major trauma, small vessel vasculitis as well as acute liver, kidney, brain, and lung injury. Finally, histones prevent the degradation of extracellular DNA, which promotes autoimmunization, anti-nuclear antibody formation, and autoimmunity in susceptible individuals. Here, we review the current evidence on the pathogenic role of extracellular histones in disease and discuss how to _target extracellular histones to improve disease outcomes.

Similar content being viewed by others
References
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Felsenfeld G, Groudine M (2003) Controlling the double helix. Nature 421:448–453
Grunstein M (1997) Histone acetylation in chromatin structure and transcription. Nature 389:349–352
Helin K, Dhanak D (2013) Chromatin proteins and modifications and drug _targets. Nature 502:480–488
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361:1570–1583
Wickman GR, Julian L, Mardlovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumptom D, MOrrice N et al (2013) Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 20:1293–1305
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Douda DN, Yip L, Khan MA, Grasemann H, Palaniyar N (2014) Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 123:597–600
Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC et al (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18:1386–1393
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD et al (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13:463–469
Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15:623–625
Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR (2013) Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A 110:1851–1856
Fink SL, Cookson BT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8:1812–1825
Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I et al (2014) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–514
Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC (2014) IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343:428–432
Linkermann A, Green D (2014) Mechanisms of disease: necroptosis. N Engl J Med 370:455–465
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW et al (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072
Yang WS, Sriramaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331
Hirsch JG (1958) Bactericidal action of histone. J Exp Med 108:925–944
Lee DY, Huang CM, Nakatsuji T, Thiboutot D, Kang SA, Monestier M, Gallo RL (2009) Histone H4 is a major component of the antimicrobial action of human sebocytes. J Invest Dermatol 129:2489–2496
Wang Y, Chen Y, Xin L, Beverley SM, Carlsen ED, Popov V, Chang KP, Wang M, Soong L (2011) Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect Immun 79:1124–1133
Rose FR, Bailey K, Keyte JW, Chan WC, Greenwood D, Mahida YR (1998) Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defence in the human gastrointestinal tract. Infect Immun 66:3255–3263
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT (2009) Extracellular histones are major mediators of death in sepsis. Nat Med 15:1318–1321
Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ (2012) Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg 73:1389–1394
Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hagele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C et al (2012) Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 23:1375–1388
Gillrie MR, Lee K, Gowda DC, Davis SP, Monestier M, Cui L, Hien TT, Day NP, Ho M (2012) Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am J Pathol 180:1028–1039
Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT (2012) Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7:e32366
Gilthorpe JD, Oozeer F, Nash J, Calvo M, Bennett DL, Lumsden A, Pini A (2013) Extracellular histone H1 is neurotoxic and drives a pro-inflammatory response in microglia. F1000Res 2: 148. DOI 10.12688/f1000research.2-148.v1
Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT (2011) Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 118:1952–1961
Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT (2011) Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 187:2626–2631
Rock KL, Latz E, Ontiveros F, Kono H (2010) The sterile inflammatory response. Ann Rev Immunol 28(28):321–342
Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT et al (2011) Endogenous histones function as alarmins in sterile inflammatory liver injury through toll-like receptor 9. Hepatology. doi:10.1002/hep.24501
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832
Allam R, Darisipudi MN, Tschopp J, Anders HJ (2013) Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol. doi:10.1002/eji.201243224
Darisipudi MN, Thomasova D, Mulay SR, Brech D, Noessner E, Liapis H, Anders HJ (2012) Uromodulin triggers IL-1beta-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol 23:1783–1789
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD (2013) Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A 110:8674–8679
Fuchs TA, Bhandari AA, Wagner DD (2011) Histones induce rapid and profound thrombocytopenia in mice. Blood 118:3708–3714
Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE (2013) Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res 132:69–76
Carestia A, Rivadeneyra L, Romaniuk MA, Fondevila C, Negrotto S, Schattner M (2013) Functional responses and molecular mechanisms involved in histone-mediated platelet activation. Thromb Haemost 110:1035–1045
Mortensen ES, Fenton KA, Rekvig OP (2008) Lupus nephritis: the central role of nucleosomes revealed. Am J Pathol 172:275–283
Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A (2010) Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107:9813–9818
Lech M, Anders HJ (2013) The pathogenesis of lupus nephritis. J Am Soc Nephrol 24:1357–1366
Mortensen ES, Rekvig OP (2009) Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol 20:696–704
Balicki D, Beutler E (1997) Histone H2A significantly enhances in vitro DNA transfection. Mol Med 3:782–787
Wildhagen KC, de Frutos GP, Reutelingsperger CP, Schrijver R, Areste C, Ortega-Gomez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA (2013) Non-anticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. doi:10.1182/blood-2013-07-514984
Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W et al (2013) Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 187:160–169
Johansson PI, Windelov NA, Rasmussen LS, Sorensen AM, Ostrowski SR (2013) Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J Emerg Trauma Shock 6:171–175
Nakahara M, Ito T, Kawahara K, Yamamoto M, Nagasato T, Shrestha B, Yamada S, Miyauchi T, Higuchi K, Takenaka T et al (2013) Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One 8:e75961
De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD (2012) Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 32:1884–1891
Pemberton AD, Brown JK (2010) In vitro interactions of extracellular histones with LDL suggest a potential pro-atherogenic role. PLoS One 5:e9884
Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA (2013) Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J 27:5010–5021
Barrero CA, Perez-Leal O, Aksoy M, Moncada C, Ji R, Lopez Y, Mallilankaraman K, Madesh M, Criner GJ, Kelsen SG et al (2013) Histone 3.3 participates in a self-sustaining cascade of apoptosis that contributes to the progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 188:673–683
Wen Z, Liu Y, Li F, Ren F, Chen D, Li X, Wen T (2013) Circulating histones exacerbate inflammation in mice with acute liver failure. J Cell Biochem 114:2384–2391
Huang H, Chen HW, Evankovich J, Yan W, Rosborough BR, Nace GW, Ding Q, Loughran P, Beer-Stolz D, Billiar TR et al (2013) Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol 191:2665–2679
Rosin DL, Okusa MD (2012) Dying cells and extracellular histones in AKI: beyond a NET effect? J Am Soc Nephrol 23:1275–1277
Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, Kirou KA, Hellmich B, Knuckley B, Thompson PR et al (2012) Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum 64:982–992
Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F, Boscaro F, Panza F, Puxeddu I, Rovero P et al (2013) Antibodies from patients with rheumatoid arthritis _target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis. doi:10.1136/annrheumdis-2012-202765
Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, Wright J, Thornhill TS, Monestier M, Ploegh H et al (2009) A broad screen for _targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A 106:15867–15872
Dwivedi N, Radic M (2013) Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis. doi:10.1136/annrheumdis-2013-203844
Fuchs TA, Alvarez JJ, Martinod K, Bhandari AA, Kaufman RM, Wagner DD (2013) Neutrophils release extracellular DNA traps during storage of red blood cell units. Transfusion 53:3210–3216
Schimmel M, Nur E, Biemond BJ, van Mierlo GJ, Solati S, Brandjes DP, Otten HM, Schnog JJ, Zeerleder S (2013) Nucleosomes and neutrophil activation in sickle cell disease painful crisis. Haematologica 98:1797–1803
Shin SH, Joo HW, Kim MK, Kim JC, Sung YK (2012) Extracellular histones inhibit hair shaft elongation in cultured human hair follicles and promote regression of hair follicles in mice. Exp Dermatol 21:956–958
Bosch X (2011) Systemic lupus erythematosus and the neutrophil. N Engl J Med 365:758–760
Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, Bengtsson AA, Blom AM (2012) Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 188:3522–3531
Migliorini A, Anders HJ (2012) A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nat Rev Nephrol 8:183–189
Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR (2012) Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 122:2661–2671
Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S (1993) Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol Immunol 30:1069–1075
Zeerleder S, Stephan F, Emonts M, de Kleijn ED, Esmon CT, Varadi K, Hack CE, Hazelzet JA (2012) Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit Care Med 40:3224–3229
Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A et al (2012) Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366:2055–2064
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107:15880–15885
Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT (2011) Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 9:1795–1803
Acknowledgement
A.R and H.J.A. were supported by the Deutsche Forschungsgemeinschaft GRK 1202. HJA by AN372/14-1.
Financial disclosure statement
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allam, R., Kumar, S.V.R., Darisipudi, M.N. et al. Extracellular histones in tissue injury and inflammation. J Mol Med 92, 465–472 (2014). https://doi.org/10.1007/s00109-014-1148-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1148-z