Abstract
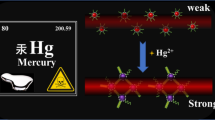
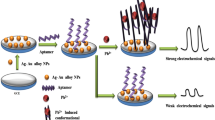
Due to heavy metals’ magnified pollution from their accumulation in the ecosystem, practical detection of ultra-low concentration of heavy metals in environmental sample is of great significance for environmental supervision and maintenance of people’s health. Herein, a practical and sensitive assay of heavy metal mercury was developed by visually observing (or spectrum detecting) the change of cationic gold nanoparticles (AuNPs), which is directly caused by mercury ion induced hybridization between non-canonical base pairs. In this assay, signal probe’s response was direct rather than the indirect salt induction, thus avoiding the defect of salt-induced indirect response. It makes the analysis more sensitive. The results showed that the response of 8.2 × 10−8 M Hg2+ could be observed with naked eye and the detection limit of Hg2+ in spectrometric determination was 4.9 × 10−11 M, which is more than one order of magnitude lower than that from indirect response pattern of signal probe. In addition, high specificity of the affinity chemistry for T–Hg–T renders the assay to be highly selective. Compared with the results of cold vapor atom adsorption spectroscopy (CVAAS), this analysis has good reliability for the detection of mercury. The results fully indicate that the developed assay is an ideal alternative for online detection of heavy metal mercury in environmental pollution samples.







Similar content being viewed by others
References
Hoyle I, Handy RD. Dose-dependent inorganic mercury absorption by isolated perfused intestine of rainbow trout, oncorhynchus mykiss, involves both amiloride-sensitive and energy-dependent pathways. Aquat Toxicol. 2005;72(1–2):147–59.
Yan N, Zhu Z, Jin L, Guo W, Gan Y, Hu S. Quantitative characterization of gold nanoparticles by coupling thin layer chromatography with laser ablation inductively coupled plasma mass spectrometry. Anal Chem. 2015;87(12):6079–87.
Otero-Romaní J, Moreda-Piñ A, Bermejo-Barrera P. Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination. Anal Chim Acta. 2005;536(1):213–8.
Madden JT, Fitzgerald N. Investigation of ultraviolet photolysis vapor generation with in-atomizer trapping graphite furnace atomic absorption spectrometry for the determination of mercury. Spectrochim Acta Part B. 2009;64(9):925–7.
Kristian KE, Friedbauer S, Kabashi D, Ferencz KM, Kelly O. A simplified digestion protocol for the analysis of Hg in fish by cold vapor atomic absorption spectroscopy. J Chem Educ. 2015;92(4):698–702.
Gao C, Wang Q, Gao F. A high-performance aptasensor for mercury (II) based on the formation of a unique ternary structure of aptamer-Hg2+-neutral red. Chem Commun. 2014;50:9397–400.
Xuan F, Luo X, Hsing I. Conformation-dependent exonuclease III activity mediated by metal ions reshuffling on thymine-rich DNA duplexes for an ultrasensitive electrochemical method for Hg2+ detection. Anal Chem. 2013;85(9):4586–93.
Chen G, Jin Y, Wang L, Deng J, Zhang CX. Gold nanorods-based FRET assay for ultrasensitive detection of Hg2+. Chem Commun. 2011;47(46):12500–2.
Ono A, Togashi H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew Chem Int Ed. 2004;43(33):4300–2.
Sun B, Jiang XX, Wang HY, Song B, Zhu Y, Wang H, et al. A surface-enhancement Raman scattering sensing strategy for discriminating trace mercuric ion (II) from real water samples in sensitive, specific, recyclable and reproducible manners. Anal Chem. 2015;87(2):1250–6.
Qi YY, Xiu FR, Yu GD, Huang LL, Li BX. Simple and rapid chemiluminescence aptasensor for Hg2+ in contaminated samples: a new signal amplification mechanism. Biosens Bioelectron. 2017;87:439–46.
Zhang M, Ge L, Ge S, Yan M, Yu J, Huang J, et al. Three-dimensional paper-based electrochemiluminescence device for simultaneous detection of Pb2+ and Hg2+ based on potential-control technique. Biosens Bioelectron. 2013;41:544–50.
Li L, Li BX, Qi YY, Yan J. Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal Bioanal Chem. 2009;393(8):2051–7.
Yan ZQ, Xue HT, Berning K, Lam YW. Identification of multi-functional graphene-gold nanocomposite for environment-friendly enriching, separating and detecting Hg2+ simultaneously. ACS Appl Mater Interfaces. 2014;6(24):22761–8.
Ding N, Zhao H, Peng W, He Y, Zhou Y, Yuan L, et al. A simple colorimetric sensor based on anti-aggregation of gold nanoparticles for Hg2+ detection. Colloid Surface. 2012;395:161–7.
Kreibig U, Genzel L. Optical absorption of small metallic particles. Surf Sci. 1985;156:678–700.
Li H, Rothberg L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. P Natl Acad Sci USA. 2004;101(39):14036–9.
Wang F, Wu YG, Zhan SS, He L, Zhi WT, Zhou XX, et al. A simple and sensitive colorimetric detection of silver ions based on cationic polymer-directed AuNPs aggregation. Aust J Chem. 2013;66(1):113–8.
Zhang L, Xu CL, Liu CW, Li BX. Visual chiral recognition of tryptophan enantiomers using unmodified gold nanoparticles as colorimetric probes. Anal Chim Acta. 2014;809:123–7.
Deng CY, Liu H, Zhang MM, Deng HH, Lei CY, Shen L, et al. Light-up nonthiolated aptasensor for low-mass, soluble amyloid β40 oligomers at high salt concentrations. Anal Chem. 2018;90:1710–7.
Qi YY, He JH, Xiu FR, Yu X, Li YF, Lu YW, et al. A convenient chemiluminescence detection for bisphenol A in E-waste dismantling site based on surface charge change of cationic gold nanoparticles. Microchem J. 2019;147:789–96.
Chen Z, Tan L, Hu L, Zhang Y, Wang S. Real colorimetric thrombin aptasensor by masking surfaces of catalytically active gold nanoparticles. ACS Appl Mater Interfaces. 2016;8(1):102–8.
Chen SJ, Huang YF, Huang CC, Lee KH, Lin ZH, Chang HT. Colorimetric determination of urinary adenosine using aptamer-modified gold nanoparticles. Biosens Bioelectron. 2008;23(11):1749–53.
Zhao W, Chiuman W, Lam JCF, McManus SA, Chen W, Cui Y. DNA aptamer folding on gold nanoparticles: from colloid chemistry to biosensors. J Am Chem Soc. 2008;130(11):3610–8.
Wu ZS, Lu H, Liu X, Hu R, Zhou H, Shen G, et al. Inhibitory effect of _target binding on hairpin aptamer sticky-end pairing-induced gold nanoparticle assembly for light-up colorimetric protein assay. Anal Chem. 2010;82(9):3890–8.
Niidome T, Nakashima K, Takahashi H, Niidome Y. Preparation of primary amine-modified gold nanoparticles and their transfection ability into cultivated cells. Chem Commun. 2004;10(17):1978–9.
Li T, Liang G, Li X. Chemiluminescence assay for the sensitive detection of iodide based on extracting Hg2+ from a T-Hg2+-T complex. Analyst. 2013;138(6):1898–902.
Qi YY, He JH, Xiu FR, Li YF, Lu YW, Gao X, et al. A facile chemiluminescence sensing for ultrasensitive detection of heparin using charge effect of positively-charged AuNPs. Spectrochim Acta Part A. 2019;216:310–8.
Qi L, Wang J, Yi H. Selective chemiluminescent sensor for detection of mercury(II) ions using non-aggregated luminol-capped gold nanoparticles. Sensors Actuators B Chem. 2016;231:64–9.
Funding
This work was supported financially by the National Natural Science Foundation of China (No. 21605018), the Natural Science Basic Research Project of Shaanxi Province of China (No. 2018JM5149), and the Foundation Research Project of Shaanxi Provincial Key Laboratory of Geological Support for Coal Green Exploitation (No. MTy2019-05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 210 kb)
Rights and permissions
About this article
Cite this article
Qi, Y., Ma, J., Chen, X. et al. Practical aptamer-based assay of heavy metal mercury ion in contaminated environmental samples: convenience and sensitivity. Anal Bioanal Chem 412, 439–448 (2020). https://doi.org/10.1007/s00216-019-02253-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02253-8