Abstract
Purpose
LY3022859 is an anti-TGFβRII IgG1 monoclonal antibody that inhibits receptor-mediated signaling activation. The primary objective of this phase I study was to determine a phase II dose in patients with advanced solid tumors. Secondary objectives were to assess safety and pharmacokinetics (PK).
Methods
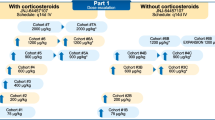
LY3022859 was infused intravenously (IV) at 1.25 mg/kg over 1 h every 2 weeks (Q2W) (cohort 1A) and at flat doses of 12.5 mg (cohort 1B) and 25 mg (cohort 2) over 3 h Q2W.
Results
Fourteen patients were enrolled in cohorts 1A (n = 2), 1B (n = 5), and 2 (n = 7). DLTs were experienced by both patients in cohort 1A (infusion-related reaction) and 2 patients in cohort 2 (cytokine release syndrome and infusion-related reaction). No MTD was determined. At the 25 mg dose level (cohort 2), after fifth infusion, LY3022859 had a short t1/2 (4.37-7.80 h) and rapid clearance (CLss, 0.412 L/h). Exposure increased twofold (from 28.5 to 60.2 μg·h/mL) with increase in dose from 12.5 to 25 mg. No accumulation was observed after repeat administration.
Conclusions
The MTD for LY3022859 was not determined. Dose escalation beyond 25 mg was considered unsafe due to worsening symptoms (uncontrolled cytokine release) despite prophylaxis (corticosteroids and antihistamines).
Trial registration
clinicaltrials.gov Identifier: NCT01646203.


Similar content being viewed by others
References
Massagué J (2008) TGFβ in cancer. Cell 134:215–230
Wrzesinski SH, Wan YY, Flavell RA (2007) Transforming growth factor-β and the immune response: implications for anticancer therapy. Clin Cancer Res 13:5262–5270
Elliott RL, Blobe GC (2005) Role of transforming growth factor beta in human cancer. J Clin Oncol 23:2078–2093
Inman GJ (2011) Switching TGFB from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev 21:93–99
Teicher BA (2007) Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res 13:6247–6251
Levy L, Hill CS (2006) Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17:41–58
Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P (2010) The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 10:554–567
Prud’homme GJ (2007) Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest 87:1077–1091
Massagué J, Gomis RR (2006) The logic of TGFβ signaling. FEBS Lett 580:2811–2820
Zhong Z, Carroll KD, Policarpio D, Osborn C, Gregory M, Bassi R, Jimenez X, Prewett M, Liebisch G, Persaud K, Burtrum D, Wang S, Surguladze D, Ng S, Griffith H, Balderes P, Doody J, Schwartz JD, Youssoufian H, Rowinsky EK, Ludwig DL, Witte L, Zhu Z, Wu Y (2010) Anti–transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res 16:1191–1205
Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapartra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I, Zaaroor M, Jachimczak P, Ludwig S, Schmaus S, Heinrichs H, Schlingensiepen KH; Trabedersen Glioma Study Group (2011) _targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13:132–142
Morris JC, Shapiro GI, Tan AR, Lawrence DP, Olencki TE, Dezube BJ, Hsu FJ, Reiss M, Berzofsky JA (2008) Phase I/II study of GC1008: a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC). J Clin Oncol 26(15 suppl):abstract 9028
Ahnert J, Baselga J, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly A, Lahn MMF, Pillay S, Holdhoff M, Blakeley JO, Carducci MA (2011) First human dose (FHD) study of the oral transforming growth factor-beta receptor I kinase inhibitor LY2157299 in patients with treatment-refractory malignant glioma. J Clin Oncol 29(15 suppl):abstract 3011
Lonning S, Mannick J, McPherson JM (2011) Antibody _targeting of TGF-β in cancer patients. Curr Pharm Biotechnol 12:2176–2189
Oettle H, Hilbig A, Seufferlein T, Tsianakas A, Luger T, Schmid RM, von Wichert G, Endlicher E, Garbe C, Kaehler KK, Hauschild A, Enk A, Kiessling P, Schmaus S, Heinrichs H, Schlingensiepen K (2011) Phase I/II study with trabedersen (AP 12009) monotherapy for the treatment of patients with advanced pancreatic cancer, malignant melanoma, and colorectal carcinoma. J Clin Oncol 29(15 suppl):abstract 2513
Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, Silliman N, Streisand J, Powell J, Akesson A, Coppock J, Hoogen Fv, Herrick A, Mayes MD, Veale D, Haas J, Ledbetter S, Korn JH, Black CM, Seibold JR; Cat-192 Study Group; Scleroderma Clinical Trials Consortium (2007) Recombinant human anti-transforming growth factor β1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 56:323–333
Lahn M, Kloeker S, Berry BS (2005) TGF-beta inhibitors for the treatment of cancer. Expert Opin Investig Drugs 14:629–643
Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JP, Streisand JB, Hard ML, Ledbetter SR, Vincenti F (2011) A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79:1236–1243
Acknowledgements
We thank David Schaer (Eli Lilly and Company) for useful discussions during the development of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding support
The study was sponsored by Eli Lilly and Company. Jude Richard (INC Research, Austin, TX) provided medical writing services on behalf of the authors, funded by the study sponsor. These included assisting with initial drafting of the manuscript and subsequent revision according to guidance from the authors.
Conflict of interest
JC was an employee of Lilly during the conduct of the study but is now an employee of Merck KGaA. EC has served on advisory boards for Lilly, Taiho, Bayer, EMD Serono, Amgen, Advaxis, Merrimack, Castle Biosciences, and Genentech. JK, ST, KD, RK, SRPK, and IG are employees of Lilly. The remaining authors declare no conflict of interest.
Ethical approval
This study was conducted in accordance with principles of the Declaration of Helsinki and Good Clinical Practice guidelines and with local ethics committee approval and was registered (NCT01646203).
Informed consent
Written informed consent was obtained from all patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tolcher, A.W., Berlin, J.D., Cosaert, J. et al. A phase 1 study of anti-TGFβ receptor type-II monoclonal antibody LY3022859 in patients with advanced solid tumors. Cancer Chemother Pharmacol 79, 673–680 (2017). https://doi.org/10.1007/s00280-017-3245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3245-5