Abstract
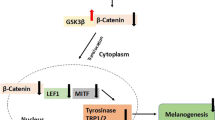
This study investigated the anti-melanogenic effect of aromatic (ar)-turmerone on alpha-melanocyte stimulating hormone (α-MSH) and 3-isobuty-1-methxlzanthine (IBMX)-induced tyrosinase (Tyr), tyrosinase-related protein 1 (TRP-1), and tyrosinase-related protein 2 (TRP-2) expression in B16F10 melanoma cells. We demonstrated that ar-turmerone inhibits α-MSH and IBMX-induced melanin synthesis and tyrosinase activity. Data also showed that ar-turmerone inhibits the expression of tyrosinase, TRP-1, and TRP-2 in α-MSH- and IBMX-stimulated B16F10 cells. In addition, ar-turmerone exhibits stronger anti-melanogenic effects than curcumin. Furthermore, ar-turmerone strongly inhibited α-MSH- and IBMX-induced microphthalmia-associated transcription factor by suppressing the activity of cyclic adenosine monophosphate (cAMP)-responsive element binding protein in α-MSH-stimulated B16F10 cells. Our data revealed that ar-turmerone is a novel, effective, anti-melanogenic agent that functions by downregulating tyrosinase, Trp-1, and Trp-2 gene expression. Therefore, ar-turmerone may be a useful therapeutic agent for treating hyperpigmentation disorders, such as freckles and melasma, and as a beneficial additive in whitening cosmetics.




Similar content being viewed by others
References
Aggarwal BB, Harikumar KB (2009) Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 41:40–59
Ahn SJ, Koketsu M, Ishihara H, Lee SM, Ha SK, Lee KH, Kang TH, Kima SY (2006) Regulation of melanin synthesis by selenium-containing carbohydrates. Chem Pharm Bull (Tokyo) 54:281–286
Bentley NJ, Eisen T, Goding CR (1994) Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol 14:7996–8006
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R (1998) Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 142:827–835
Boissy RE (2003) Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol 12(Suppl 2):5–12
Busca R, Bertolotto C, Ortonne JP, Ballotti R (1996) Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J Biol Chem 271:31824–31830
Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M (1996) Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta 1313:130–138
Costin GE, Hearing VJ (2007) Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 21:976–994
Eller MS, Ostrom K, Gilchrest BA (1996) DNA damage enhances melanogenesis. Proc Natl Acad Sci USA 93:1087–1092
Eves PC, MacNeil S, Haycock JW (2006) Alpha-melanocyte stimulating hormone, inflammation and human melanoma. Peptides 27:444–452
Gescher A (2004) Polyphenolic phytochemicals versus non-steroidal anti-inflammatory drugs: which are better cancer chemopreventive agents? J Chemother 16(Suppl 4):3–6
Goding CR (2000) Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev 14:1712–1728
Ha SK, Koketsu M, Lee K, Choi SY, Park JH, Ishihara H, Kim SY (2005) Inhibition of tyrosinase activity by N,N-unsubstituted selenourea derivatives. Biol Pharm Bull 28:838–840
Halaban R, Pomerantz SH, Marshall S, Lerner AB (1984) Tyrosinase activity and abundance in cloudman melanoma cells. Arch Biochem Biophys 230:383–387
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV (2008) Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65:1631–1652
Hunt G, Todd C, Cresswell JE, Thody AJ (1994) Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci 107(Pt 1):205–211
Jang JY, Lee JH, Jeong SY, Chung KT, Choi YH, Choi BT (2009) Partially purified Curcuma longa inhibits alpha-melanocyte-stimulating hormone-stimulated melanogenesis through extracellular signal-regulated kinase or Akt activation-mediated signalling in B16F10 cells. Exp Dermatol 18:689–694
Jung E, Lee J, Huh S, Lee J, Kim YS, Kim G, Park D (2009) Phloridzin-induced melanogenesis is mediated by the cAMP signaling pathway. Food Chem Toxicol 47:2436–2440
Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA (2003) Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res 16:434–447
Kanwar AJ, Dhar S, Kaur S (1994) Treatment of melasma with potent topical corticosteroids. Dermatology 188:170
Kubo I, Kinst-Hori I, Chaudhuri SK, Kubo Y, Sanchez Y, Ogura T (2000) Flavonols from Heterotheca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg Med Chem 8:1749–1755
Mas JS, Gerritsen I, Hahmann C, Jimenez-Cervantes C, Garcia-Borron JC (2003) Rate limiting factors in melanocortin 1 receptor signalling through the cAMP pathway. Pigment Cell Res 16:540–547
Mountjoy KG, Robbins LS, Mortrud MT, Cone RD (1992) The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251
Ohguchi K, Ito M, Yokoyama K, Iinuma M, Itoh T, Nozawa Y, Akao Y (2009) Effects of sesquiterpene lactones on melanogenesis in mouse B16 melanoma cells. Biol Pharm Bull 32:308–310
Perez-Gilabert M, Garcia-Carmona F (2001) Dimethyl sulfide, a volatile flavor constituent, is a slow-binding inhibitor of tyrosinase. Biochem Biophys Res Commun 285:257–261
Riley PA (2003) Melanogenesis and melanoma. Pigment Cell Res 16:548–552
Roh JS, Han JY, Kim JH, Hwang JK (2004) Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol Pharm Bull 27:1976–1978
Sassone-Corsi P (1995) Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol 11:355–377
Unver N, Freyschmidt-Paul P, Horster S, Wenck H, Stab F, Blatt T, Elsasser HP (2006) Alterations in the epidermal-dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. Br J Dermatol 155:119–128
Virador V, Matsunaga N, Matsunaga J, Valencia J, Oldham RJ, Kameyama K, Peck GL, Ferrans VJ, Vieira WD, Abdel-Malek ZA, Hearing VJ (2001) Production of melanocyte-specific antibodies to human melanosomal proteins: expression patterns in normal human skin and in cutaneous pigmented lesions. Pigment Cell Res 14:289–297
Wong G, Pawelek J (1975) Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in cloudman S91 melanoma cells. Nature 255:644–646
Yang JY, Koo JH, Song YG, Kwon KB, Lee JH, Sohn HS, Park BH, Jhee EC, Park JW (2006) Stimulation of melanogenesis by scoparone in B16 melanoma cells. Acta Pharmacol Sin 27:1467–1473
Yasumoto K, Yokoyama K, Takahashi K, Tomita Y, Shibahara S (1997) Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J Biol Chem 272:503–509
Yokota T, Nishio H, Kubota Y, Mizoguchi M (1998) The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res 11:355–361
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S.Y., Jin, M.L., Kim, Y.H. et al. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch Dermatol Res 303, 737–744 (2011). https://doi.org/10.1007/s00403-011-1155-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-011-1155-7