Abstract
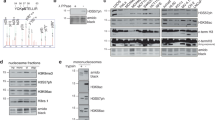
The cell cycle-associated phosphorylation of histone H1.5 is manifested as three discrete phosphorylated forms, occurring exclusively on Ser17, Ser172, and Ser188 during interphase. During late G2 and mitosis the up-phosphorylation occurs exclusively on threonine at either Thr137 or Thr154 to build the tetraphosphorylated forms of H1.5, whereas the pentaphosphorylated forms result from phosphorylation at Thr10. To determine the kinetic and spatial distribution of histone H1 phosphorylation within the nucleus of synchronized Hela cells we localized three distinct phosphorylation sites of histone subtype H1.5 using affinity-purified polyclonal antibodies generated against phosphorylated Ser17, Ser172, and Thr10. Immunofluorescence labeling of synchronized HeLa cells using the specific antibodies revealed that phosphorylation of H1.5 Ser17 appeared early in G1 at discrete speckles followed by phosphorylation of Ser172. Thr10 phosphorylation started during prophase, showed highest phosphorylation levels during metaphase, and disappeared clearly before chromatin decondensation occurred. Experiments using the kinase inhibitor staurosporine indicate the involvement of different kinases at the various phospho-sites. Colocalization studies revealed that Ser172 phosphorylation of H1.5 and H1.2 does colocalize to DNA replication and transcription sites. These results favor the idea that the various site-specifically phosphorylated forms of H1.5 and H1.2 localized at distinct regions of the nucleus are related to different functions during the cell cycle.











Similar content being viewed by others
References
Albig W, Meergans T, Doenecke D (1997) Characterization of the H1.5 gene completes the set of human H1 subtype genes. Gene 184:141–148
Alexandrow MG, Hamlin JL (2005) Chromatin decondensation in S-phase involves recruitment of Cdk2 by Cdc45 and histone H1 phosphorylation. J Cell Biol 168:875–886
Banks GC, Deterding LJ, Tomer KB, Archer TK (2001) Hormone-mediated dephosphorylation of specific histone H1 isoforms. J Biol Chem 276:36467–36473
Bhan S, May W, Warren SL, Sittman DB (2008) Global gene expression analysis reveals specific and redundant roles for H1 variants, H1c and H1(0), in gene expression regulation. Gene 414:10–18
Bleher R, Martin R (1999) Nucleo-cytoplasmic translocation of histone H1 during the HeLa cell cycle. Chromosoma 108:308–316
Breneman JW, Yau P, Teplitz RL, Bradbury EM (1993) A light microscope study of linker histone distribution in rat metaphase chromosomes and interphase nuclei. Exp Cell Res 206:16–26
Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A (2008) Detection of S-phase cell cycle progression using 5-ethynyl-2'-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2'-deoxyuridine antibodies. Biotechniques 44:927–929
Bustin M, Catez F, Lim JH (2005) The dynamics of histone H1 function in chromatin. Mol Cell 17:617–620
Cao G, Liu LM, Cleary SF (1991) Modified method of mammalian cell synchronization improves yield and degree of synchronization. Exp Cell Res 193:405–410
Chadee DN, Taylor WR, Hurta RA, Allis CD, Wright JA, Davie JR (1995) Increased phosphorylation of histone H1 in mouse fibroblasts transformed with oncogenes or constitutively active mitogen-activated protein kinase kinase. J Biol Chem 270:20098–20105
Chen D, Dundr M, Wang C, Leung A, Lamond A, Misteli T, Huang S (2005) Condensed mitotic chromatin is accessible to transcription factors and chromatin structural proteins. J Cell Biol 168:41–54
Dimitrova DS, Berezney R (2002) The spatio-temporal organization of DNA replication sites is identical in primary, immortalized and transformed mammalian cells. J Cell Sci 115:4037–4051
Dou Y, Gorovsky MA (2000) Phosphorylation of linker histone H1 regulates gene expression in vivo by creating a charge patch. Mol Cell 6:225–231
Dou Y, Mizzen CA, Abrams M, Allis CD, Gorovsky MA (1999) Phosphorylation of linker histone H1 regulates gene expression in vivo by mimicking H1 removal. Mol Cell 4:641–647
Gorka C, Fakan S, Lawrence JJ (1993) Light and electron microscope immunocytochemical analyses of histone H1(0) distribution in the nucleus of Friend erythroleukemia cells. Exp Cell Res 205:152–158
Halmer L, Gruss C (1995) Influence of histone H1 on the in vitro replication of DNA and chromatin. Nucleic Acids Res 23:773–778
Happel N, Stoldt S, Schmidt B, Doenecke D (2008) M-phase-specific phosphorylation of histone H1.5 at threonine 10 by GSK-3. J Mol Biol 386:339–350
Hassan AB, Errington RJ, White NS, Jackson DA, Cook PR (1994) Replication and transcription sites are colocalized in human cells. J Cell Sci 107:425–434
Hellauer K, Sirard E, Turcotte B (2001) Decreased expression of specific genes in yeast cells lacking histone H1. J Biol Chem 276:13587–13592
Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360
Jackson DA, Hassan AB, Errington RJ, Cook PR (1993) Visualization of focal sites of transcription within human nuclei. EMBO J 12:1059–1065
Koop R, Di Croce L, Beato M (2003) Histone H1 enhances synergistic activation of the MMTV promoter in chromatin. EMBO J 22:588–599
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lennox RW, Cohen LH (1988) The production of tissue-specific histone complements during development. Biochem Cell Biol 66:636–649
Lever MA, Th'ng JP, Sun X, Hendzel MJ (2000) Rapid exchange of histone H1.1 on chromatin in living human cells. Nature 408:873–876
Lindner H, Sarg B, Helliger W (1997) Application of hydrophilic-interaction liquid chromatography to the separation of phosphorylated H1 histones. J Chromatogr A 782:55–62
Lu MJ, Dadd CA, Mizzen CA, Perry CA, McLachlan DR, Annunziato AT, Allis CD (1994) Generation and characterization of novel antibodies highly selective for phosphorylated linker histone H1 in Tetrahymena and HeLa cells. Chromosoma 103:111–121
Lu MJ, Mpoke SS, Dadd CA, Allis CD (1995) Phosphorylated and dephosphorylated linker histone H1 reside in distinct chromatin domains in Tetrahymena macronuclei. Mol Biol Cell 6:1077–1087
Misteli T, Gunjan A, Hock R, Bustin M, Brown DT (2000) Dynamic binding of histone H1 to chromatin in living cells. Nature 408:877–881
Nakamura H, Morita T, Sato C (1986) Structural organizations of replicon domains during DNA synthetic phase in the mammalian nucleus. Exp Cell Res 165:291–297
Nakayasu H, Berezney R (1989) Mapping replicational sites in the eucaryotic cell nucleus. J Cell Biol 108:1–11
Parseghian MH, Hamkalo BA (2001) A compendium of the histone H1 family of somatic subtypes: an elusive cast of characters and their characteristics. Biochem Cell Biol 79:289–304
Parseghian MH, Harris DA, Rishwain DR, Hamkalo BA (1994) Characterization of a set of antibodies specific for three human histone H1 subtypes. Chromosoma 103:198–208
Parseghian MH, Newcomb RL, Winokur ST, Hamkalo BA (2000) The distribution of somatic H1 subtypes is non-random on active vs. inactive chromatin: distribution in human fetal fibroblasts. Chromosome Res 8:405–424
Parseghian MH, Newcomb RL, Hamkalo BA (2001) Distribution of somatic H1 subtypes is non-random on active vs. inactive chromatin II: distribution in human adult fibroblasts. J Cell Biochem 83:643–659
Roque A, Ponte I, Arrondo JL, Suau P (2008) Phosphorylation of the carboxy-terminal domain of histone H1: effects on secondary structure and DNA condensation. Nucleic Acids Res 36:4719–4726
Roth SY, Allis CD (1992) Chromatin condensation: does histone H1 dephosphorylation play a role? Trends Biochem Sci 17:93–98
Salic A, Mitchison TJ (2008) A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 105:2415–2420
Sancho M, Diani E, Beato M, Jordan A (2008) Depletion of human histone H1 variants uncovers specific roles in gene expression and cell growth. PLoS Genet 4:e1000227
Sarg B, Helliger W, Talasz H, Forg B, Lindner HH (2006) Histone H1 phosphorylation occurs site-specifically during interphase and mitosis: identification of a novel phosphorylation site on histone H1. J Biol Chem 281:6573–6580
Sarg B, Chwatal S, Talasz H, Lindner HH (2009) Testis-specific linker histone H1t is multiply phosphorylated during spermatogenesis: identification of phosphorylation sites. J Biol Chem 284:3610–3618
Shen X, Gorovsky MA (1996) Linker histone H1 regulates specific gene expression but not global transcription in vivo. Cell 86:475–483
Sweet MT, Jones K, Allis CD (1996) Phosphorylation of linker histone is associated with transcriptional activation in a normally silent nucleus. J Cell Biol 135:1219–1228
Takami Y, Nishi R, Nakayama T (2000) Histone H1 variants play individual roles in transcription regulation in the DT40 chicken B cell line. Biochem Biophys Res Commun 268:501–508
Talasz H, Helliger W, Puschendorf B, Lindner H (1996) In vivo phosphorylation of histone H1 variants during the cell cycle. Biochemistry 35:1761–1767
Talasz H, Sapojnikova N, Helliger W, Lindner H, Puschendorf B (1998) In vitro binding of H1 histone subtypes to nucleosomal organized mouse mammary tumor virus long terminal repeat promotor. J Biol Chem 273:32236–32243
Thiriet C, Hayes JJ (2008) Linker histone phosphorylation regulates global timing of replication origin firing. J Biol Chem 284:2823–2829
Vermaak D, Steinbach OC, Dimitrov S, Rupp RA, Wolffe AP (1998) The globular domain of histone H1 is sufficient to direct specific gene repression in early Xenopus embryos. Curr Biol 8:533–536
Wei X, Somanathan S, Samarabandu J, Berezney R (1999) Three-dimensional visualization of transcription sites and their association with splicing factor-rich nuclear speckles. J Cell Biol 146:543–558
Acknowledgment
We thank L. Sattler and Dr. M. Rittinger for their excellent technical assistance. This work, as part of the European Science Foundation EUROCORES Program EuroDYNA, was supported by funds from the Austrian Science Foundation (project I23-B03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.A. Nigg
Rights and permissions
About this article
Cite this article
Talasz, H., Sarg, B. & Lindner, H.H. Site-specifically phosphorylated forms of H1.5 and H1.2 localized at distinct regions of the nucleus are related to different processes during the cell cycle. Chromosoma 118, 693–709 (2009). https://doi.org/10.1007/s00412-009-0228-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-009-0228-2