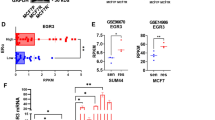
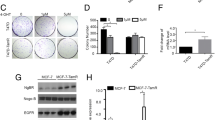
Abstract
Breast cancer (BC) is a worldwide malignant and a leading death cancer in women. Studies have shown that adjuvant tamoxifen reduces the recurrence rate and metastasis in BC. Even though tamoxifen has been used for the therapy of BC for decades, the resistance of it on BC cells could not be ignored. In this study, we first established a tamoxifen-resistant BC cell line and then demonstrated the overexpression of nuclear envelope integral membrane protein 1 (NEMP1) in the tamoxifen-resistant BC cells. Moreover, through a cell viability assay combined with depletion or overexpression technology, we addressed the important role of NEMP1 for the tamoxifen resistance in BC cells. Importantly, we further revealed that NEMP1 modulated tamoxifen resistance by regulating nuclear receptor coactivator 1 (NCOA1). In general, NEMP1 shows responsibility for the resistance of tamoxifen through regulating NCOA1 in BC cells. These results broaden the understanding of the tamoxifen resistance during the chemotherapy in BC and may provide new therapy method for BC.





Similar content being viewed by others
References
Clarke R et al (2003) Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 22:7316–7339. https://doi.org/10.1038/sj.onc.1206937
Coughlin SS, Ekwueme DU (2009) Breast cancer as a global health concern. Cancer Epidemiol 33:315–318. https://doi.org/10.1016/j.canep.2009.10.003
Dowsett M et al (2005) Growth factor signalling and response to endocrine therapy: the Royal Marsden Experience. Endocr Relat Cancer 12:S113–S117. https://doi.org/10.1677/erc.1.01044
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. https://doi.org/10.1016/s0140-6736(05)66544-0
Early Breast Cancer Trialists’ Collaborative Group et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. https://doi.org/10.1016/s0140-6736(11)60993-8
Gonzalez-Sistal A, Sanchez AB, Del Rio MC, Arias JI, Herranz M, Ruibal A (2014) Association between tumor size and immunohistochemical expression of Ki-67, p53 and BCL2 in a node-negative breast cancer population selected from a breast cancer screening program. Anticancer Res 34:269–273
Hayes DF (2004) Tamoxifen: Dr. Jekyll and Mr. Hyde? J Natl Cancer Inst 96:895–897
He L et al (2018) Imbalance of the reciprocally inhibitory loop between the ubiquitin-specific protease USP43 and EGFR/PI3K/AKT drives breast carcinogenesis. Cell Res. https://doi.org/10.1038/s41422-018-0079-6
Iacobas DA, Tuli NY, Iacobas S, Rasamny JK, Moscatello A, Geliebter J, Tiwari RK (2018) Gene master regulators of papillary and anaplastic thyroid cancers. Onco_target 9:2410–2424. https://doi.org/10.18632/onco_target.23417
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300. https://doi.org/10.3322/caac.20073
Jeruss JS et al (2008) Staging of breast cancer in the neoadjuvant setting. Can Res 68:6477–6481. https://doi.org/10.1158/0008-5472.Can-07-6520
Jia M, Dahlman-Wright K, Gustafsson JA (2015) Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 29:557–568. https://doi.org/10.1016/j.beem.2015.04.008
Jiang Z et al (2017) miRNA-214 inhibits cellular proliferation and migration in glioma cells _targeting caspase 1 involved in pyroptosis. Oncol Res 25:1009–1019. https://doi.org/10.3727/096504016x14813859905646
Kechagioglou P et al (2014) Tumor suppressor PTEN in breast cancer: heterozygosity, mutations and protein expression. Anticancer Res 34:1387–1400
Krishnamurti U, Silverman JF (2014) HER2 in breast cancer: a review and update. Adv Anat Pathol 21:100–107. https://doi.org/10.1097/pap.0000000000000015
Li H et al (2014) Phosphatidylethanolamine-binding protein 4 is associated with breast cancer metastasis through Src-mediated Akt tyrosine phosphorylation. Oncogene 33:4589–4598. https://doi.org/10.1038/onc.2013.408
Lu PW, Li L, Wang F, Gu YT (2018) Inhibitory role of large intergenic noncoding RNA-ROR on tamoxifen resistance in the endocrine therapy of breast cancer by regulating the PI3K/Akt/mTOR signaling pathway. J Cell Physiol 10:10. https://doi.org/10.1002/jcp.27066
Luef B et al (2016) The AR/NCOA1 axis regulates prostate cancer migration by involvement of PRKD1. Endocr Relat Cancer 23:495–508. https://doi.org/10.1530/erc-16-0160
Mamada H, Takahashi N, Taira M (2009) Involvement of an inner nuclear membrane protein, Nemp1, in Xenopus neural development through an interaction with the chromatin protein BAF. Dev Biol 327:497–507. https://doi.org/10.1016/j.ydbio.2008.12.038
Meerson A, Yehuda H (2016) Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer 16:882. https://doi.org/10.1186/s12885-016-2938-1
Musgrove EA, Sutherland RL (2009) Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9:631–643. https://doi.org/10.1038/nrc2713
Normanno N et al (2005) Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer 12:721–747. https://doi.org/10.1677/erc.1.00857
Notas G et al (2015) Tamoxifen induces a pluripotency signature in breast cancer cells and human tumors. Mol Oncol 9:1744–1759. https://doi.org/10.1016/j.molonc.2015.05.008
Nwachukwu JC et al (2016) Predictive features of ligand-specific signaling through the estrogen receptor. Mol Syst Biol 12:864. https://doi.org/10.15252/msb.20156701
Qin L et al (2011) Steroid receptor coactivator-1 upregulates integrin alpha(5) expression to promote breast cancer cell adhesion and migration. Cancer Res 71:1742–1751. https://doi.org/10.1158/0008-5472.can-10-3453
Qin L et al (2014) NCOA1 directly _targets M-CSF1 expression to promote breast cancer metastasis. Cancer Res 74:3477–3488. https://doi.org/10.1158/0008-5472.can-13-2639
Qin L et al (2015) NCOA1 promotes angiogenesis in breast tumors by simultaneously enhancing both HIF1alpha- and AP-1-mediated VEGFa transcription. Onco_target 6:23890–23904. https://doi.org/10.18632/onco_target.4341
Redmond AM et al (2009) Coassociation of estrogen receptor and p160 proteins predicts resistance to endocrine treatment; SRC-1 is an independent predictor of breast cancer recurrence. Clin Cancer Res 15:2098–2106. https://doi.org/10.1158/1078-0432.ccr-08-1649
Shi L et al (2009) Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol 27:3423–3429. https://doi.org/10.1200/jco.2008.17.2254
Shibano T, Mamada H, Hakuno F, Takahashi S, Taira M (2015) The inner nuclear membrane protein Nemp1 is a new type of RanGTP-binding protein in eukaryotes. PLoS ONE 10:e0127271. https://doi.org/10.1371/journal.pone.0127271
Simpson ER et al (2005) Estrogen—the good, the bad, and the unexpected. Endocr Rev 26:322–330. https://doi.org/10.1210/er.2004-0020
Terunuma A et al (2014) MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Investig 124:398–412. https://doi.org/10.1172/jci71180
Tomczak K, Czerwinska P, Wiznerowicz M (2015) The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn) 19:A68–A77. https://doi.org/10.5114/wo.2014.47136
Tyson JJ et al (2011) Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer 11:523–532. https://doi.org/10.1038/nrc3081
Ullah Shah A, Mahjabeen I, Kayani MA (2015) Genetic polymorphisms in cell cycle regulatory genes CCND1 and CDK4 are associated with susceptibility to breast cancer. J BUON 20:985–993
Zhang K et al (2018) Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res 10:2573–2580. https://doi.org/10.2147/cmar.s173193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Tong, C., Cao, J. et al. NEMP1 Promotes Tamoxifen Resistance in Breast Cancer Cells. Biochem Genet 57, 813–826 (2019). https://doi.org/10.1007/s10528-019-09926-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-019-09926-0