Abstract
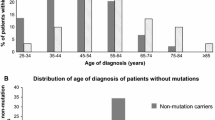
Approximately 10% of Ashkenazi Jewish (AJ) women with breast cancer (BC) carry a founder mutation in BRCA1 or BRCA2. There is an association between BRCA1 mutations and “triple-negative” breast cancer (TNBC) [estrogen receptor (ER) and progesterone receptor (PR) negative, HER2 negative]. We sought to determine the predictive value of the TNBC phenotype for the presence of a BRCA mutation in AJ women ascertained without respect to family history. DNA samples were collected between 8/2000 and 6/2004 from a prevalent cohort of unselected AJ women with breast cancer (median age at diagnosis 56 years). Samples (n = 451) were genotyped for AJ founder mutations. 352 (78.0%) cancers were ER positive, 254 (56.3%) PR positive, and 91 (20.2%) ER negative/PR negative. 63 (14.0%) cancers were HER2 positive (immunohistochemistry 3+ or FISH >2.2). TNBC was observed in 64 patients (14.2%). Founder mutations were detected in 48 samples (10.6%) including 25/64 TNBC (39.1%; 19 BRCA1, 6 BRCA2). Among TNBC patients with family history (FH) information, 6/15 (40%) mutations were found in women without breast or ovarian cancer in a close relative. The positive predictive value of TNBC for a BRCA1 mutation was 30% overall, 50% in women diagnosed<50 years, and 14% in women diagnosed ≥50. TNBC was significantly associated with detecting a mutation in either BRCA1 or BRCA2, but only 25/52 (48%) mutation-associated cancers were TNBC. The prevalence of BRCA founder mutations exceeds 50% in subsets of AJ women with TNBC. FH is an imperfect predictor of mutation status in this group. A significant number of mutation-associated TNBC are due to BRCA2.
Similar content being viewed by others
References
Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JC, Lashkari D, Shalon D, Brown PO, Botstein D (1999) Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA 96(16):9212–9217
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol 26(15):2568–2581. doi:10.1200/JCO.2007.13.1748
Turner NC, Reis-Filho JS (2006) Basal-like breast cancer and the BRCA1 phenotype. Oncogene 25(43):5846–5853. doi:10.1038/sj.onc.1209876
Turner N, Tutt A, Ashworth A (2004) Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 4(10):814–819. doi:10.1038/nrc1457
Evans DG, Lalloo F, Cramer A, Jones EA, Knox F, Amir E, Howell A (2009) Addition of pathology and biomarker information significantly improves the performance of the Manchester scoring system for BRCA1 and BRCA2 testing. J Med Genet 46(12):811–817. doi:10.1136/jmg.2009.067850
Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA (1997) The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336(20):1401–1408. doi:10.1056/NEJM199705153362001
Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Begin LR, Offit K, Foulkes WD (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 6(1):R8–R17. doi:10.1186/bcr658
Zhang L, Kirchhoff T, Yee CJ, Offit K (2009) A rapid and reliable test for BRCA1 and BRCA2 founder mutation analysis in paraffin tissue using pyrosequencing. J Mol Diagn 11(3):176–181. doi:10.2353/jmoldx.2009.080137
Newcombe RG (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17(8):857–872
Wilson EB (1927) Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 22(158):209–212
Kwon JS, Gutierrez-Barrera AM, Young D, Sun CC, Daniels MS, Lu KH, Arun B (2010) Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol 28(27):4214–4220. doi:10.1200/JCO.2010.28.0719
Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton J, Potter J, Lanchbury JS, Stemke-Hale KA, Hennessy B, Arun BK, Hortobagyi GN, Do K-A, Mills GB, Meric-Bernstam F (2011) Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clinical Cancer Research. doi:10.1158/1078-0432.ccr-10-2560
Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA (2003) Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 95(19):1482–1485
Karp SE, Tonin PN, Bégin LR, Martinez JJ, Zhang JC, Pollak MN, Foulkes WD (1997) Influence of BRCA1 mutations on nuclear grade and estrogen receptor status of breast carcinoma in Ashkenazi Jewish women. Cancer 80(3):435–441
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S (2010) American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6(4):195–197. doi:10.1200/JOP.777003
Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN, Arun BK (2008) Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 26(26):4282–4288. doi:10.1200/JCO.2008.16.6231
Kandel MJ, Stadler Z, Masciari S et al. (2006) Prevalence of BRCA1 mutations in triple negative breast cancer (BC). J Clin Oncol, 2006 ASCO Annual Meeting Proceedings Part I, vol 24, No. 18S (June 20 Supplement)
Distelman-Menachem T, Shapira T, Laitman Y, Kaufman B, Barak F, Tavtigian S, Friedman E (2009) Analysis of BRCA1/BRCA2 genes’ contribution to breast cancer susceptibility in high risk Jewish Ashkenazi women. Fam Cancer 8(2):127–133. doi:10.1007/s10689-008-9216-6
Laitman Y, Borsthein RT, Stoppa-Lyonnet D, Dagan E, Castera L, Goislard M, Gershoni-Baruch R, Goldberg H, Kaufman B, Ben-Baruch N, Zidan J, Maray T, Soussan-Gutman L, Friedman E (2010) Germline mutations in BRCA1 and BRCA2 genes in ethnically diverse high risk families in Israel. Breast Cancer Res Treat. doi:10.1007/s10549-010-1217-0
Kauff ND, Perez-Segura P, Robson ME, Scheuer L, Siegel B, Schluger A, Rapaport B, Frank TS, Nafa K, Ellis NA, Parmigiani G, Offit K (2002) Incidence of non-founder BRCA1 and BRCA2 mutations in high risk Ashkenazi breast and ovarian cancer families. J Med Genet 39(8):611–614
Chang J, Hilsenbeck SG, Sng JH, Wong J, Ragu GC (2001) Pathological features and BRCA1 mutation screening in premenopausal breast cancer patients. Clin Cancer Res 7(6):1739–1742
Lidereau R, Eisinger F, Champeme MH, Nogues C, Bieche I, Birnbaum D, Pallud C, Jacquemier J, Sobol H (2000) Major improvement in the efficacy of BRCA1 mutation screening using morphoclinical features of breast cancer. Cancer Res 60(5):1206–1210
Haffty BG, Choi DH, Goyal S, Silber A, Ranieri K, Matloff E, Lee MH, Nissenblatt M, Toppmeyer D, Moran MS (2009) Breast cancer in young women (YBC): prevalence of BRCA1/2 mutations and risk of secondary malignancies across diverse racial groups. Ann Oncol 20(10):1653–1659. doi:10.1093/annonc/mdp051
Young SR, Pilarski RT, Donenberg T, Shapiro C, Hammond LS, Miller J, Brooks KA, Cohen S, Tenenholz B, Desai D, Zandvakili I, Royer R, Li S, Narod SA (2009) The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Cancer 9:86. doi:10.1186/1471-2407-9-86
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comen, E., Davids, M., Kirchhoff, T. et al. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi Women. Breast Cancer Res Treat 129, 185–190 (2011). https://doi.org/10.1007/s10549-011-1433-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1433-2