Abstract
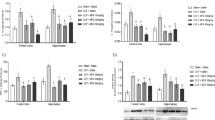
Sepsis is a life-threatening organ dysfunction, which demands notable attention for its treatment, especially in view of the involvement of immunodepressed patients, as the case of patients with diabetes mellitus (DM), who constitute a population susceptible to develop infections. Thus, considering this endocrine pathology as an implicatory role on the immune system, the aim of this study was to show the relationship between this disease and sepsis on neuroinflammatory and neurochemical parameters. Levels of IL-6, IL-10, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and mitochondrial respiratory chain complexes were evaluated in the hippocampus and prefrontal cortex 24 h after sepsis by cecal ligation and perforation (CLP) in Wistar rats induced to type 1 diabetes by alloxan (150 mg/kg). It was verified that diabetes implied immune function after 24 h of sepsis, since it contributed to the increase of the inflammatory process with higher production of IL-6 and decreased levels of IL-10 only in the hippocampus. In the same brain area, a several decrease in NGF level and activity of complexes I and II of the mitochondrial respiratory chain were observed. Thus, diabetes exacerbates neuroinflammation and results in mitochondrial impairment and downregulation of NGF level in the hippocampus after sepsis.






Similar content being viewed by others
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author, FP, upon reasonable request.
References
Trevelin, Silvia C., Daniela Carlos, Matteo Beretta, and Fernando Q. Cunha. 2017. Diabetes mellitus and sepsis: A challenging association. Shock 47: 276–287. https://doi.org/10.1097/SHK.0000000000000778.
Evans, Laura, Andrew Rhodes, Waleed Alhazzani, Massimo Antonelli, Craig M Coopersmith, Craig French, Flávia R Machado, et al. 2021. Surviving sepsis campaign : international guidelines for management of sepsis and septic shock 2021. Intensive Care Medicine 47: 1181–1247. Springer Berlin Heidelberg. https://doi.org/10.1007/s00134-021-06506-y.
Singer, Mervyn, Clifford S. Deutschman, Christopherwarren Seymour, Manu Shankar-Hari, Djillali Annane, Michael Bauer, Rinaldo Bellomo, et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA - Journal of the American Medical Association. American Medical Association. https://doi.org/10.1001/jama.2016.0287.
Sankowski, Roman, Simone Mader, and Sergio Iván Valdés-Ferrer. 2015. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Frontiers in Cellular Neuroscience 9: 1–20. https://doi.org/10.3389/fncel.2015.00028.
Biff, Daiane, Fabricia Petronilho, Larissa Constantino, Francieli Vuolo, Grettel J. Zamora-Berridi, Dhebora Mozena, and Dall’Igna, Larissa M. Comim, Joao Quevedo, Flavio Kapczinski, and Felipe Dal-Pizzol. 2013. Correlation of acute phase inflammatory and oxidative markers with long-term cognitive impairment in sepsis survivors rats. Shock 40: 45–48. https://doi.org/10.1097/SHK.0b013e3182959cfa.
Dal-Pizzol, Felipe, Cristiane D. Tomasi, and Cristiane Ritter. 2014. Septic encephalopathy: Does inflammation drive the brain crazy? Revista Brasileira de Psiquiatria 36: 251–258. https://doi.org/10.1590/1516-4446-2013-1233.
Danielski, Lucineia Gainski, Amanda Della Giustina, Marwa Badawy, Tatiana Barichello, João. Quevedo, Felipe Dal-Pizzol, and Fabrícia Petronilho. 2018. Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Molecular Neurobiology 55: 1045–1053. https://doi.org/10.1007/s12035-016-0356-7.
Fink, Mitchell P., and Stephen O. Heard. 1990. Laboratory models of sepsis and septic shock. Journal of Surgical Research 49: 186–196. https://doi.org/10.1016/0022-4804(90)90260-9.
Michels, M., L.G. Danieslki, A. Vieira, D. Florentino, D. Dall’Igna, L. Galant, B. Sonai, et al. 2015. CD40-CD40 ligand pathway is a major component of acute neuroinflammation and contributes to long-term cognitive dysfunction after sepsis. Molecular Medicine 21. https://doi.org/10.2119/molmed.2015.0
Rittirsch, D., M.S. Huber-Lang, M.A. Flierl, and P.A. Ward. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36. https://doi.org/10.1038/nprot.2008.214.
Benedet, Joana, Kozuchovski Ferreira, Pereira Teodorak, and Martinelli Comim. 2011. Inhibition of brain citrate synthase activity in an animal model of sepsis. Revista Brasileira de terapia intensiva 23: 158–163. https://doi.org/10.1590/S0103-507X2011000200007.
Lima, Lea, Socorro Ferraz, and Marta Chagas Monteiro. 2010. Fatores de risco associados ao agravamento de sepse em pacientes em Unidade de Terapia Intensiva the Intensive Care Unit. Cadernos Saúde Coletiva 24: 388–396. https://doi.org/10.1590/1414-462X201600040091.
Koh, G.C.K.W., S.J. Peacock, T. Van Der Poll, and W.J. Wiersinga. 2012. The impact of diabetes on the pathogenesis of sepsis. European Journal of Clinical Microbiology & Infectious Diseases 31: 379–388. https://doi.org/10.1007/s10096-011-1337-4.
Mantzarlis, Konstantinos, Vasiliki Tsolaki, and Epaminondas Zakynthinos. 2017. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxidative Medicine and Cellular Longevity. Hindawi Limited. https://doi.org/10.1155/2017/5985209.
Vieira, A., M. Michels, D. Florentino, A.A. Lauriano, L.G. Danielski, J.J. Fortunato, T. Barichello, D.-P. Felipe, J. Quevedo, and F. Petronilho. 2014. Increased on oxidative brain injury in the diabetic rats following sepsis. Synapse 68. https://doi.org/10.1002/syn.21753
Kottaisamy, Chidhambara Priya Dharshini, Divya S. Raj, V. Prasanth Kumar, and Umamaheswari Sankaran. 2021. Experimental animal models for diabetes and its related complications-a review. Laboratory animal research 37. https://doi.org/10.1186/S42826-021-00101-4.
Sun, Jian, Jingxiao Zhang, Jiakun Tian, Grazia Maria Virzì, Kumar Digvijay, Laura Cueto, Yongjie Yin, Mitchell H. Rosner, and Claudio Ronco. 2019. Mitochondria in sepsis-induced AKI. Journal of the American Society of Nephrology 30: 1151–1161. https://doi.org/10.1681/ASN.2018111126.
Zhang, Hui, Yong Wen Feng, and Yong Ming Yao. 2018. Potential therapy strategy: _targeting mitochondrial dysfunction in sepsis. Military Medical Research 5: 1–11. https://doi.org/10.1186/s40779-018-0187-0.
Belenguer, Pascale, João M.N. Duarte, Patrícia F. Schuck, and Gustavo C. Ferreira. 2019. Mitochondria and the brain: bioenergetics and beyond. Neurotoxicity research 36: 219–238. https://doi.org/10.1007/S12640-019-00061-7.
Shenoy, Santosh. 2020. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflammation Research. Springer Science and Business Media Deutschland GmbH. https://doi.org/10.1007/s00011-020-01389-z.
Wasyluk, Weronika, and Agnieszka Zwolak. 2021. Metabolic alterations in sepsis. Journal of Clinical Medicine 10: 1–18. https://doi.org/10.3390/jcm10112412.
Calvo-Ochoa, Erika, Karina Hernández-Ortega, Patricia Ferrera, Sumiko Morimoto, and Clorinda Arias. 2014. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34: 1001–1008. https://doi.org/10.1038/JCBFM.2014.48.
Dutheil, Sophie, Kristie T. Ota, Eric S. Wohleb, Kurt Rasmussen, and Ronald S. Duman. 2016. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41: 1874–1887. Neuropsychopharmacology. https://doi.org/10.1038/NPP.2015.357.
Li, Yuanzhe, Huayan Zhao, Yalin Guo, Yongtao Duan, Yanjun Guo, and Xianfei Ding. 2021. Association of preadmission metformin use and prognosis in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis. Frontiers in endocrinology 12: 811776. Frontiers Media S.A. https://doi.org/10.3389/fendo.2021.811776.
Yao, Weifeng, Haofeng Liao, Mengya Pang, Lijie Pan, Yu Guan, Xiaolei Huang, Ziqing Hei, Chenfang Luo, and Mian Ge. 2022. Inhibition of the NADPH oxidase pathway reduces ferroptosis during septic renal injury in diabetic mice. Edited by Liang-Jun Yan. Oxidative medicine and cellular longevity 2022: 1193734. https://doi.org/10.1155/2022/1193734.
Cassina, Adriana, and Rafael Radi. 1996. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Archives of Biochemistry and Biophysics 328: 309–316. https://doi.org/10.1006/abbi.1996.0178.
Fischer, Johan C., Wim Ruitenbeek, Jan A. Berden, J.M. Frans Trijbels, Jacques H. Veerkamp, Ad.M. Stadhouders, Rob C.A.. Sengers, and Antoon J.M.. Janssen. 1985. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clinica Chimica Acta 153: 23–36. https://doi.org/10.1016/0009-8981(85)90135-4.
Dal-Pizzol, Felipe, Cristiane Ritter, Omar J. Cassol-Jr, Gislaine T. Rezin, Fabrícia Petronilho, Alexandra I. Zugno, João. Quevedo, and Emilio L. Streck. 2010. Oxidative mechanisms of brain dysfunction during sepsis. Neurochemical Research 35: 1–12. https://doi.org/10.1007/s11064-009-0043-4.
Bermpohl, Daniela, Annett Halle, Dorette Freyer, Emilie Dagand, Johann S. Braun, Ingo Bechmann, Nicolas W.J.. Schröder, and Joerg R. Weber. 2005. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. Journal of Clinical Investigation 115: 1607–1615. https://doi.org/10.1172/JCI23223.
Cancelier, Ana Carolina, Fabricia Petronilho, Adalisa Reinke, Larissa Constantino, Roberta Machado, Cristiane Ritter, and Felipe Dal-Pizzol. 2009. Inflammatory and oxidative parameters in cord blood as diagnostic of early-onset neonatal sepsis: A case-control study. Pediatric critical care medicine : A journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 10: 467–471. https://doi.org/10.1097/PCC.0b013e318198b0e3.
Bozza, Fernando A., Joana C. D’Avila, Cristiane Ritter, Romain Sonneville, Tarek Sharshar, and Felipe Dal-Pizzol. 2013. Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 39: 10–16. https://doi.org/10.1097/SHK.0b013e31828fade1.
Peleg, Anton Y., Thilak Weerarathna, James McCarthy, and Timothy M.E.. Davis. 2007. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes/Metabolism Research and Reviews 23: 3–13. https://doi.org/10.1002/dmrr.
Minelli, Lorivaldo, Andrei Bungart Nonino, Jaqueline Cantarelli Salmazo, Leandro Neme, and Marcelo Marcondes. 2003. Diabetes mellitus and cutaneous affections. Anais Brasileiros de Dermatologia 78: 735–747. https://doi.org/10.1590/s0365-05962003000600010.
Berbudi, Afiat, Nofri Rahmadika, Adi Imam Tjahjadi, and Rovina Ruslami. 2020. Type 2 diabetes and its impact on the immune system. Current Diabetes Reviews 16: 442–449. https://doi.org/10.2174/1573399815666191024085838.
Frydrych, Lynn M., Fatemeh Fattahi, Katherine He, Peter A. Ward, Matthew J. Delano, and Matthew J. Delano. 2017. Diabetes and sepsis : Risk, recurrence, and ruination. Frontiers in Endocrinology 8: 1–22. https://doi.org/10.3389/fendo.2017.00271.
Plummer, Mark P., and Adam M. Deane. 2016. Dysglycemia and glucose control during sepsis. Clinics in Chest Medicine 37: 309–319. https://doi.org/10.1016/j.ccm.2016.01.010.
Feketeova, Eleonora, Zhifeng Li, Biju Joseph, Roshan Shah, Zoltan Spolarics, and Luis Ulloa. 2018. Dopaminergic control of inflammation and glycemia in sepsis and diabetes. Frontiers in Immunology 9: 1–12. https://doi.org/10.3389/fimmu.2018.00943.
Branco, Ricardo Garcia, Robert Charles Tasker, Pedro Celiny, Ramos Garcia, Jefferson Pedro Piva, and Lisandra Dias Xavier. 2007. Glycemic control and insulin therapy in sepsis and critical illness 83: 128–136. https://doi.org/10.2223/JPED.1710.
Fabbri, Andrea, Giulio Marchesini, Barbara Benazzi, Alice Morelli, Danilo Montesi, Cesare Bini, and Stefano Giovanni Rizzo. 2020. Stress hyperglycemia and mortality in subjects with diabetes and sepsis. Critical Care Explorations 2: 1–6. https://doi.org/10.1097/CCE.0000000000000152.
Decarlo, Kristen, and Amisha Wallia. 2019. Inpatient management of T2DM and hyperglycemia in older adults. Current Diabetes Reports 19: 104.
Akinosoglou, Karolina, Georgia Kapsokosta, Maria Mouktaroudi, Nikoletta Rovina, Vassileios Kaldis, Aggelos Stefos, and Evangelos Giamarellos-bourboulis. 2021. Diabetes on sepsis outcomes in non-ICU patients: a cohort study and review of the literature. Journal of Diabetes and Its Complications 35: 107765. Elsevier Inc. https://doi.org/10.1016/j.jdiacomp.2020.107765.
Dewilde, Stephen, Tess Harris, Fay J. Hosking, and Derek G. Cook. 2018. Risk of infection in type 1 and type 2 diabetes compared with the general population : A matched cohort study. Diabetes Care 41: 513–521. https://doi.org/10.2337/dc17-2131/-/DC1.This.
Wasyluk, Weronika, Martyna Wasyluk, and Agnieszka Zwolak. 2021. Sepsis as a pan-endocrine illness — endocrine disorders in septic patients. Journal of Clinical Medicine 10: 1–14. https://doi.org/10.3390/jcm10102075.
Annane, Djillali, and Tarek Sharshar. 2015. Cognitive decline after sepsis. The Lancet Respiratory Medicine 3: 61–69. https://doi.org/10.1016/S2213-2600(14)70246-2.
Monje, Michelle L., Hiroki Toda, and Theo D. Palmer. 2003. Inflammatory blockade restores adult hippocampal neurogenesis. Science 302: 1760–1765. https://doi.org/10.1126/science.1088417.
Semmler, Alexander, Catherine Nichols Widmann, Thorsten Okulla, Horst Urbach, Markus Kaiser, Guido Widman, Florian Mormann, et al. 2013. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. Journal of Neurology, Neurosurgery and Psychiatry 84: 62–70. BMJ Publishing Group. https://doi.org/10.1136/jnnp-2012-302883.
Schwalm, Mágada T, Matheus Pasquali, Samantha P Miguel, João Paulo, and A Santos. 2013. Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats: 13–18. https://doi.org/10.1007/s12035-013-8526-3.
Lou, H.C. 1996. Etiology and pathogenesis of attention-deficit hyperactivity disorder (ADHD): Significance of prematurity and perinatal hypoxic-haemodynamic encephalopathy. Acta Paediatrica, International Journal of Paediatrics 85: 1266–1271. https://doi.org/10.1111/j.1651-2227.1996.tb13909.x.
Chousterman, Benjamin G, Filip K Swirski, and Georg F Weber. 2017. Cytokine storm and sepsis disease pathogenesis. Seminars in Immunopathology 39: 517–528. https://doi.org/10.1007/s00281-017-0639-8.
Ishihara, Katsuhiko, and Toshio Hirano. 2002. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine & Growth Factor Reviews 13: 357–368. https://doi.org/10.1016/s1359-6101(02)00027-8.
Łukaszewicz, Marta, Barbara Mroczko, and Maciej Szmitkowski. 2007. Clinical significance of interleukin-6 (IL-6) as a prognostic factor of cancer disease. Polskie Archiwum Medycyny Wewnetrznej 117: 247–51. https://doi.org/10.20452/pamw.144.
Maraschin, Jorge De, Nádia Murussi. Faria, Vanessa Witter, and Sandra Pinho Silveiro. 2010. Atualização Clínica Classificação do Diabete Melito. Arquivos Brasileiros de Cardiologia 95: 40–47.
Kellum, John A., Lan Kong, Mitchell P. Fink, Lisa A. Weissfeld, Donald M. Yealy, Michael R. Pinsky, Jonathan Fine, Alexander Krichevsky, Russell L. Delude, and Derek C. Angus. 2007. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Archives of Internal Medicine 167: 1655–1663. https://doi.org/10.1001/archinte.167.15.1655.Understanding.
Kumar, Anil T., U. Sudhir, K. Punith, V.N. Rahul Kumar, Ravi Kumar, and Medha Y. Rao. 2008. Cytokine profile in elderly patients with sepsis. Indian Journal of Critical Care Medicine 13: 74–78. https://doi.org/10.4103/0972-5229.56052.
Feng, Mingchen, Tingting Sun, Yaxin Zhao, and Hui Zhang. 2016. Detection of serum interleukin-6/10/18 levels in sepsis and its clinical significance. Journal of Clinical Laboratory Analysis 30: 1037–1043. https://doi.org/10.1002/jcla.21977.
Michels, Monique, Mariane Rocha, Abatti Pricila, Ávila. Andriele, Heloisa Borges, Celso Carvalho, Junior Diogo, et al. 2020. Characterization and modulation of microglial phenotypes in an animal model of severe sepsis. Journal of Cellular and Molecular Medicine 24: 88–97. https://doi.org/10.1111/jcmm.14606.
Nolasco, Eduardo Lima. 2013. Estudo da sepse experimental em animais diabéticos e sadios , tratados ou não com insulina. Universidade de São Paulo.
Vieira, Andriele, Monique Michels, Drielly Florentino, André Antunes. Lauriano, Lucineia Gainski Danielski, Jucelia Jeremias Fortunato, Tatiana Barichello, Dal Pizzol Felipe, Joao Quevedo, and Fabricia Petronilho. 2014. Increased on oxidative brain injury in the diabetic rats following sepsis. Synapse (New York, N. Y.) 68: 410–418. https://doi.org/10.1002/syn.21753.
Forrest, Shelley L., Jillian J. Kril, and Glenda M. Halliday. 2019. Cellular and regional vulnerability in frontotemporal tauopathies. Acta Neuropathologica. Springer Verlag. https://doi.org/10.1007/s00401-019-02035-7.
Vasic, Verica, and Mirko H H. Schmidt. 2017. Resilience and vulnerability to pain and inflammation in the hippocampus. International Journal of Molecular Sciences Review 18: 2–14. https://doi.org/10.3390/ijms18040739.
Petronilho, Fabricia. 2012. Marcadores inflamatórios e oxidativos em sangue de cordão umbilical como preditores de gravidade em sepse neonatal. Revista Brasileira de Terapia Intensiva 24: 30–34. https://doi.org/10.1590/S0103-507X2012000100005.
Asadullah, K., W. Sterry, and H.D. Volk. 2003. Interleukin-10 therapy — review of a new approach. Pharmacological Reviews 55: 241–269. https://doi.org/10.1124/pr.55.2.4.241.
Mohammadi, Mola, Homa Manaheji, Nader Maghsoudi, Samira Danyali, Mansoureh Baniasadi, and Jalal Zaringhalam. 2020. Microglia dependent BDNF and proBDNF can impair spatial memory performance during persistent inflammatory pain. Behavioural Brain Research 390: 112683. https://doi.org/10.1016/j.bbr.2020.112683.
Florentino, Drielly, Amanda Della Giustina, Mariana Pereira de Souza Goldim, Lucineia Gainski Danielski, Aloir Neri de Oliveira Junior, Larissa Joaquim, Sandra Bonfante, et al. 2020. Early life neuroimmune challenge protects the brain after sepsis in adult rats. Neurochemistry International 135: 104712. Elsevier. https://doi.org/10.1016/j.neuint.2020.104712.
Cassol-Jr, Omar J., Clarissa M. Comim, Larissa S. Constantino, Daniela V.F. Rosa, Luiz Alexandre V. Mango, Laura Stertz, Flávio Kapczinski, Marco A. Romano-Silva, João Quevedo, and Felipe Dal-Pizzol. 2011. Acute low dose of MK-801 prevents memory deficits without altering hippocampal DARPP-32 expression and BDNF levels in sepsis survivor rats. Journal of Neuroimmunology 230: 48–51. Elsevier B.V. https://doi.org/10.1016/j.jneuroim.2010.08.026.
Martínez-Levy, Gabriela A., and Carlos S. Cruz-Fuentes. 2014. Genetic and epigenetic regulation of the brain-derived neurotrophic factor in the central nervous system. Yale Journal of Biology and Medicine 87: 173–186.
Lipsky, Robert H., and Ann M. Marini. 2007. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Annals of the New York Academy of Sciences 1122: 130–143. https://doi.org/10.1196/annals.1403.009.
Minnone, Gaetana, Fabrizio De Benedetti, and Luisa Bracci-laudiero. 2017. NGF and its receptors in the regulation of inflammatory response. https://doi.org/10.3390/ijms18051028.
Chandra, Mondal Amal, and Fatima Mahino. 2018. Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. International Journal of Neuroscience 129: 283–296. Taylor & Francis. https://doi.org/10.1080/00207454.2018.1527328.
Espliguero, Gemma, Teresa Iglesias, and Angeles Rodrı. 2004. Expression of the neurotrophin receptor trkB is regulated by the cAMP / CREB pathway in neurons. Molecular and Cellular Neuroscience 26: 470–480. https://doi.org/10.1016/j.mcn.2004.03.007.
Venkatesan, Ramu, Eunhee Ji, and Sun Yeou Kim. 2015. Phytochemicals that regulate neurodegenerative disease by _targeting neurotrophins : A comprehensive review. BioMed Research International 2015: 1–22. https://doi.org/10.1155/2015/814068.
Spencer, Jeremy P E, David Vauzour, and Catarina Rendeiro. 2009. Flavonoids and cognition : the molecular mechanisms underlying their behavioural effects. Archives of Biochemistry and Biophysics 492: 1–9. Elsevier Inc. https://doi.org/10.1016/j.abb.2009.10.003.
Pugazhenthi, Subbiah, Ketaki Phansalkar, Gerald Audesirk, Anne West, and Leigh Cabell. 2006. Differential regulation of c-jun and CREB by acrolein and 4-hydroxynonenal. Free Radical Biology and Medicine 40: 21–34. https://doi.org/10.1016/j.freeradbiomed.2005.08.023.
Wu, Aiguo, Z.H.E. Ying, and Fernando Gomez-pinilla. 2004. Disability after traumatic brain injury in rats. Journal of Neurotrauma 21: 1457–1467.
Zou, Jian, and Fulton Crews. 2006. CREB and NF-κB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and Molecular Neurobiology 26: 385–405. https://doi.org/10.1007/s10571-006-9045-9.
Della Giustina, Amanda, Mariana Pereira Goldim, Lucinéia Gainski Danielski, Drielly Florentino, Khiany Mathias, Leandro Garbossa, Aloir Neri Oliveira Junior, et al. 2017. Alpha-lipoic acid attenuates acute neuroinflammation and long-term cognitive impairment after polymicrobial sepsis. Neurochemistry International 108: 436–447. Elsevier Ltd. https://doi.org/10.1016/j.neuint.2017.06.003.
Goldstein, Benjamin I., Katelyn A. Collinger, Francis Lotrich, Anna L. Marsland, Mary-Kay. Gill, David A. Axelson, and Boris Birmaher. 2011. Preliminary findings regarding proinflammatory markers and brain-derived neurotrophic factor among adolescents with bipolar spectrum disorders. Journal of child and adolescent psychopharmacology 21: 479–484. https://doi.org/10.1089/cap.2011.0009.
Barichello, Tatiana, Jaqueline S. Generoso, Lutiana R. Simões, Amanda V. Steckert, Ana Paula Moreira, Diogo Dominguini, Pâmela. Ferrari, et al. 2015. Folic acid prevented cognitive impairment in experimental pneumococcal meningitis. Journal of Neural Transmission 122: 643–651. https://doi.org/10.1007/s00702-014-1302-3.
Blondeau, Nicolas, Carine Nguemeni, David N. Debruyne, Marie Piens, Wu. Xuan, Hongna Pan, Hu. Xian Zhang, et al. 2009. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: A versatile potential therapy for stroke. Neuropsychopharmacology 34: 2548–2559. https://doi.org/10.1038/npp.2009.84.
Pudell, Claudia, Bianca A. Vicente, Ana M. Delattre, Bruno Carabelli, Marco A. Mori, Deborah Suchecki, Ricardo B. Machado, et al. 2014. Fish oil improves anxiety-like, depressive-like and cognitive behaviors in olfactory bulbectomised rats. European Journal of Neuroscience 39: 266–274. https://doi.org/10.1111/ejn.12406.
Bavaresco, Daniela V, Luciane B Ceretta, Karin M Gomes, and Graziela Amboni. 2016. Prejuízos cognitivos em Diabetes Mellitus: revisão da literatura. Prejuízos cognitivos em Diabetes Mellitus: revisão da literatura 5: 30–41. https://doi.org/10.18616/is.v5i1.2336.
de Almeida-Pititto, Bianca, M. de Clineu, Almada Filho, and Maysa S. Cendoroglo. 2008. Déficit cognitivo: Mais uma complicação do diabetes melito? Arquivos Brasileiros de Endocrinologia & Metabologia 52: 1076–1083. https://doi.org/10.1590/s0004-27302008000700003.
Craft, Suzanne, and G. Stennis Watson. 2004. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurology 3: 169–178. https://doi.org/10.1016/S1474-4422(04)00681-7.
Cheng, Aiwu, Yan Hou, and Mark P. Mattson. 2010. Mitochondria and neuroplasticity. ASN Neuro 2: 243–256. https://doi.org/10.1042/AN20100019.
Marosi, Krisztina, and Mark P. Mattson. 2014. BDNF mediates adaptive brain and body responses to energetic challenges. Trends in Endocrinology and Metabolism 25: 89–98. Elsevier Ltd. https://doi.org/10.1016/j.tem.2013.10.006.
Li, Longman, and Xiaobo Yang. 2018. The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Edited by Pan Chen. Oxidative Medicine and Cellular Longevity 2018: 7580707. Hindawi. https://doi.org/10.1155/2018/7580707.
Candas, Demet, and Jian Jian Li. 2014. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxidants & Redox Signaling 20: 1599–1617. https://doi.org/10.1089/ars.2013.5305.
Acknowledgements
The authors would like to thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico -CNPq).
Funding
This research was supported by grants from the Santa Catarina State Foundation for Research Support (FAPESC) and National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq). FP, ES, and GTR are CNPq Research Fellow. The funding sources were not involved in the conduction of the research, preparation of the article nor in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
SSS, MH, and FP conceived, designed, and coordinated the experiments and manuscript preparation. LGD, SB, LJ, EB, EL, GB, and AP performed the diabetes and sepsis induction. TD, TC, MPO, LES, and GTR performed the biochemical analyses. JMM and FB performed the ELISA analyses. SSS, TB, and MH analyzed the data, prepared the figures, and wrote the manuscript. FP and ES revised and edited the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
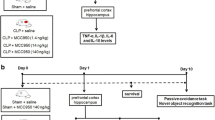
This study was approved by the Animal Research Ethic Committee of the University of Southern Santa Catarina (protocol 12.027.4.03.IV). The “Principles of Laboratory Animal Care” (NIH publication n° 80–23, revised 1996) and the “EC Directive 86/609/EEC” were followed in all experiments.
Consent for Publication
All authors gave their consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza Stork, S., Hübner, M., Biehl, E. et al. Diabetes Exacerbates Sepsis-Induced Neuroinflammation and Brain Mitochondrial Dysfunction. Inflammation 45, 2352–2367 (2022). https://doi.org/10.1007/s10753-022-01697-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01697-y