Abstract
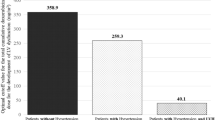
Advances in oncologic therapies have allowed to achieve better outcomes and longer survival in many patients with breast cancer. Anthracyclines are cytotoxic antibiotics widely used in daily oncology practice. However, anthracyclines cause cardiotoxicity which is a limiting factor of its use. Cumulative dose of anthracyclines is the major cause of induced cardiotoxicity. According to previous clinical trials, the major predisposing high-risk factors for anthracycline-based chemotherapy-induced cardiotoxicity are age, body weight, female gender, radiotherapy, and other diseases such as diabetes and hypertension. Experimental studies in animals confirm that hypertension may be a significant factor predisposing anthracycline-based chemotherapy cardiotoxicity. The main objective of our study was to identify the effect of pre-existing arterial hypertension on the development of subclinical cardiac damage during or after doxorubicin-based chemotherapy in breast cancer patients. The study was performed prospectively between March 2016 and January 2017 in the Hospital of Lithuanian University of Health Sciences Kaunas Clinics Department of Oncology and Department of Cardiology. Data of 73 women with breast cancer treated with doxorubicin-based chemotherapy in outpatient clinic were analyzed. Statistically significant association between pre-existing arterial hypertension and left ventricular systolic dysfunction after completion of chemotherapy was observed (P < 0.004). Our study demonstrated that pre-existing arterial hypertension has a very important role in the development of anthracycline-based chemotherapy-induced cardiotoxicity, despite arterial hypertension control quality. Consequently, further studies evaluating impact of other risk factors and how early and sufficient management of arterial hypertension could influence the development of cardiotoxicity are needed to avoid permanent cardiac damage.
Similar content being viewed by others
References
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians,68(6), 394–424. https://doi.org/10.3322/caac.21492.
Curigliano, G., Cardinale, D., Suter, T., Plataniotis, G., de Azambuja, E., Sandri, M. T., et al. (2012). Cardiovascular toxicity induced by chemotherapy, _targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Annals of Oncology,23(Suppl 7), vii155–vii166.
Rygiel, K. (2016). Benefits of antihypertensive medications for anthracycline- and trastuzumab-induced cardiotoxicity in patients with breast cancer: Insights from recent clinical trials. Indian Journal of Pharmacology,48(5), 490–497.
Szmit, S., Jurczak, W., Zaucha, J. M., Drozd-Sokołowska, J., Spychałowicz, W., Joks, M., et al. (2014). Pre-existing arterial hypertension as a risk factor for early left ventricular systolic dysfunction following (R)-CHOP chemotherapy in patients with lymphoma. Journal of the American Society of Hypertension,8(11), 791–799. https://doi.org/10.1016/j.jash.2014.08.009.
Gianni, L., Herman, E. H., Lipshultz, S. E., Minotti, G., Sarvazyan, N., & Sawyer, D. B. (2008). Anthracycline cardiotoxicity: From bench to bedside. Journal of Clinical Oncology,26(22), 3777–3784. https://doi.org/10.1200/JCO.2007.14.9401.
Arcamone, F., Franceschi, G., Penco, S., & Selva, A. (1969). Adriamycin (14-hydroxydaunomycin), a novel antitumor antibiotic. Tetrahedron Letters,13, 1007–1010.
Menna, P., Paz, O. G., Chello, M., Covino, E., Salvatorelli, E., & Minotti, G. (2012). Anthracycline cardiotoxicity. Expert Opinion on Drug Safety,11(Suppl 1), S21–S36. https://doi.org/10.1517/14740338.2011.589834.
Valcovici, M., Andrica, F., Serban, C., & Dragan, S. (2016). Cardiotoxicity of anthracycline therapy: Current perspectives. Archives of Medical Science,12(2), 428–435. https://doi.org/10.5114/aoms.2016.59270.
Seidman, A., Hudis, C., Pierri, M. K., Shak, S., Paton, V., Ashby, M., et al. (2002). Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of Clinical Oncology,20(5), 1215–1221.
Albini, A., Pennesi, G., Donatelli, F., Cammarota, R., De Flora, S., & Noonan, D. M. (2010). Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. Journal of the National Cancer Institute,102(1), 14–25. https://doi.org/10.1093/jnci/djp440.
Seidman, A., Hudis, C., Pierri, M. K., et al. (2002). Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of Clinical Oncology,20, 1215–1221.
Pinder, M. C., Duan, Z., Goodwin, J. S., Hortobagyi, G. N., & Giordano, S. H. (2007). Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. Journal of Clinical Oncology,25(25), 3808–3815.
Hershman, D. L., McBride, R. B., Eisenberger, A., Tsai, W. Y., Grann, V. R., & Jacobson, J. S. (2008). Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. Journal of Clinical Oncology,26(19), 3159–3165. https://doi.org/10.1200/JCO.2007.14.1242.
Salazar-Mendiguchía, J., González-Costello, J., Roca, J., Ariza-Solé, A., Manito, N., & Cequier, A. (2014). Anthracycline-mediated cardiomyopathy: Basic molecular knowledge for the cardiologist. Archivos de Cardiología de México,84(3), 218–223. https://doi.org/10.1016/j.acmx.2013.08.006.
Kuriakose, R. K., Kukreja, R. C., & Xi, L. (2016). Potential therapeutic strategies for hypertension-exacerbated cardiotoxicity of anticancer drugs. Oxidative Medicine and Cellular Longevity,2016, 8139861.
Mancia, G., Fagard, R., Narkiewicz, K., Redon, J., Zanchetti, A., & Böhm, M. (2014). 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Pressure,23(1), 3–16. https://doi.org/10.3109/08037051.2014.868629.
Raj, S., Franco, V. I., & Lipshultz, S. E. (2014). Anthracycline-induced cardiotoxicity: A review of pathophysiology, diagnosis, and treatment. Current Treatment Options in Cardiovascular Medicine,16(6), 315. https://doi.org/10.1007/s11936-014-0315-4.
Vivenza, D., Feola, M., Garrone, O., Monteverde, M., Merlano, M., & Lo, Nigro C. (2013). Role of the renin-angiotensin-aldosterone system and the glutathione S-transferase Mu, Pi and Theta gene polymorphisms in cardiotoxicity after anthracycline chemotherapy for breast carcinoma. International Journal of Biological Markers,28(4), e336–e347. https://doi.org/10.5301/jbm.5000041.
Aebi, S., Davidson, T., Gruber, G., Castiglione, M., & ESMO Guidelines Working Group. (2010). Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology,21(Suppl 5), v9–v14. https://doi.org/10.1093/annonc/mdq159.
Cortés-Funes, H., & Coronado, C. (2007). Role of anthracyclines in the era of _targeted therapy. Cardiovascular Toxicology,7(2), 56–60.
Swain, S. M., Whaley, F. S., & Ewer, M. S. (2003). Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of threetrials. Cancer,97(11), 2869–2879.
Eschenhagen, T., Force, T., Ewer, M. S., de Keulenaer, G. W., Suter, T. M., Anker, S. D., et al. (2011). Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure,13(1), 1–10. https://doi.org/10.1093/eurjhf/hfq213.
Von Hoff, D. D., Layard, M. W., Basa, P., Davis, H. L., Jr., Von Hoff, A. L., Rozencweig, M., et al. (1979). Risk factors for doxorubicin-induced congestive heart failure. Annals of Internal Medicine,91(5), 710–717.
Sharkey, L. C., Radin, M. J., Heller, L., Rogers, L. K., Tobias, A., Matise, I., et al. (2013). Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension-heart failure (SHHF), spontaneously hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicology and Applied Pharmacology,273(1), 47–57. https://doi.org/10.1016/j.taap.2013.08.012.
Hazari, M. S., Haykal-Coates, N., Winsett, D. W., Costa, D. L., & Farraj, A. K. (2009). Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicological Sciences,110(1), 224–234. https://doi.org/10.1093/toxsci/kfp092.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by GM, DV, GA, data collection and analysis were performed by DM, LS, DV and review was performed by AV, RJ, EJ. The first draft of the manuscript was written by Domas Vaitiekus and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
Permission from the Kaunas regional Bioethics Committee was obtained. (No.: BEC MF 361). All patients gave written informed consent.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vaitiekus, D., Muckiene, G., Vaitiekiene, A. et al. Impact of Arterial Hypertension on Doxorubicin-Based Chemotherapy-Induced Subclinical Cardiac Damage in Breast Cancer Patients. Cardiovasc Toxicol 20, 321–327 (2020). https://doi.org/10.1007/s12012-019-09556-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09556-3