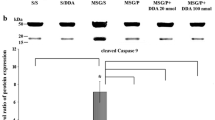
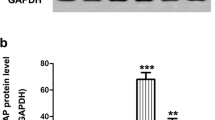
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is neuroprotective in animal models of different brain pathologies and injuries, including cerebral ischemia, Parkinson’s disease, and different types of retinal degenerations. We have previously shown that PACAP is protective against monosodium glutamate (MSG)-induced retinal degeneration, where PACAP-treated retinas has more retained structure and PACAP induces anti-apoptotic while it inhibits pro-apoptotic signaling pathways. The aim of the present study was to investigate cell-type specific effects of PACAP in MSG-induced retinal degeneration by means of immunohistochemistry. Rat pups received MSG (2 mg/g b.w.) applied on postnatal days 1, 5, and 9. PACAP (100 pmol in 5 μl saline) was injected into the right vitreous body, while the left eye received only saline. Retinas were processed for immunocytochemistry after 3 weeks. Immunolabeling was determined for vesicular glutamate transporter 1, tyrosine hydroxylase, calretinin, calbindin, parvalbumin, and vesicular γ-aminobutyric acid (GABA) transporter. In the MSG-treated retinas, the cell bodies and processes in the inner nuclear, inner plexiform, and ganglion cell layers displayed less immunoreactivity for all antisera. Apart from photoreceptors, only one major retinal cell type examined in this study; the calbindin-immunoreactive horizontal cell seemed not to be affected by MSG application. After simultaneous application of MSG and PACAP, staining of retinas was similar to that of normal eyes, with no significant alterations in immunoreactive patterns. These findings further support the neuroprotective function of PACAP in MSG-induced retinal degeneration.






Similar content being viewed by others
References
Atlasz, T., Babai, N., Kiss, P., et al. (2007). Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. General and Comparative Endocrinology, 153, 108–114.
Babai, N., Atlasz, T., Tamas, A., et al. (2006). Search for the optimal monosodium glutamate treatment schedule to study the neuroprotective effects of PACAP in the retina. Annals of the New York Academy of Sciences, 1070, 149–155.
Babai, N., Atlasz, T., Tamas, A., Reglodi, D., Kiss, P., & Gabriel, R. (2005). Degree of damage compensation by various PACAP treatments in monosodium glutamate-induced retina degeneration. Neurotoxicity Research, 8, 227–233.
Bagnoli, P., Dal Monte, M., & Casini, G. (2003). Expression of neuropeptides and their receptors in the developing retina of mammals. Histology and Histopathology, 18, 1219–1242.
Bellocchio, E. E., Reimer, R. J., Fremeau, R. T., Jr., & Edwards, R. H. (2000). Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science, 289, 957–960.
Borba, J. C., Henze, I. P., Silveira, M. S., et al. (2005). Pituitary adenylate cyclase-activating polypeptide (PACAP) can act as determinant of the tyrosine hydroxylase phenotype of dopaminergic cells during retinal development. Developmental Brain Research, 156, 193–201.
Brandstätter, J. H. (2002). Glutamate receptors in the retina: the molecular substrate for visual signal processing. Current Eye Research, 25, 327–331.
Contini, M., & Raviola, E. (2003). GABAergic synapses made by a retinal dopaminergic neuron. Proceedings of the National Academy of Sciences of the United States of America, 100, 1358–1363.
Cueva, J. G., Haverkamp, S., Reimer, R. J., Edwards, R., Wässle, H., & Brecha, N. C. (2002). Vesicular γ-aminobutiric acid transporter expression in amacrine and horizontal cells. Journal of Comparative Neurology, 445, 227–237.
Fahrenkrug, J., Nielsen, H. S., & Hannibal, J. (2004). Expression of melanopsin during development of the rat retina. Neuroreport, 15, 781–784.
Falluel-Morel, A., Chafai, M., Vaudry, D., et al. (2007). The neuropeptide pituitary adenylate cyclase activating polypeptide exerts anti-apoptotic and differentiating effects during neurogenesis: focus on cerebellar granule neurones and embryonic stem cells. Journal of Neuroendocrinology, 19, 321–327.
Gabriel, R., & Witkovsky, P. (1998). Cholinergic, but not the rod-pathway-related glycinergic (AII), amacrine cells contain calretinin in the rat retina. Neuroscience Letters, 247, 179–182.
Gong, J., Jellali, A., Mutterer, J., Sahel, J. A., Rendon, A., & Picaud, S. (2006). Distribution of vesicular glutamate transporters in rat and human retina. Brain Research, 1082, 73–85.
Gustincich, S., Contini, M., Gariboldi, M., et al. (2004). Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proceedings of the National Academy of Sciences of the United States of America, 101, 5069–5074.
Hamano, K., Kiyama, H., Emson, P. C., Manabe, R., Nakauchi, M., & Tohyama, M. (1990). Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. Journal of Comparative Neurology, 302, 417–424.
Hannibal, J., & Fahrenkrug, J. (2004). _target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell & Tissue Research, 316, 99–113.
Haverkamp, S., Grunert, U., & Wässle, H. (2000). The cone pedicle, a complex synapse in the retina. Neuron, 27, 85–95.
Johnson, J., Tian, N., Caywood, M. S., Reimer, R. J., Edwards, R. H., & Copenhagen, D. R. (2003). Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. Journal of Neuroscience, 23, 518–529.
Jozsa, R., Somogyvari-Vigh, A., Reglodi, D., Hollosy, T., & Arimura, A. (2001). Distribution and daily variations of PACAP in the chicken brain. Peptides, 22, 1371–1377.
Kiss, P., Tamas, A., Lubics, A., et al. (2006). Effects of systemic PACAP treatment in monosodium glutamate-induced behavioral changes and retinal degeneration. Annals of the New York Academy of Sciences, 1070, 365–370.
McIntire, S. L., Reimer, R. J., Schuske, K., Edwards, R. H., & Jorgensen, E. M. (1997). Identification and characterization of the vesicular GABA transporter. Nature, 389, 870–876.
Nakajima, Y., Iwakabe, H., Akazawa, C., et al. (1993). Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. Journal of Biological Chemistry, 268, 11368–11373.
Nakatani, M., Seki, T., Shinohara, Y., et al. (2006). Pituitary adenylate cyclase activating polypeptide (PACAP) stimulates production of interleukin-6 in rat Muller cells. Peptides, 27, 1871–1876.
Nilsson, S. F. (1994). PACAP-27 and PACAP-38: vascular effects in the eye and some other tissues in the rabbit. European Journal of Pharmacology, 253, 17–25.
Pasteels, B., Rogers, J., Blachier, F., & Pochet, R. (1990). Calbindin and calretinin localization in retina from different species. Visual Neuroscience, 5(1), 1–16.
Pothos, E. N., Larsen, K. E., Krantz, D. E., et al. (2000). Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. Journal of Neuroscience, 20, 7297–7306.
Racz, B., Gallyas, F., Jr., Kiss, P., et al. (2006a). The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involves inhibition of proapoptotic signaling pathways. Regulatory Peptides, 137, 20–26.
Racz, B., Gallyas, F., Jr., Kiss, P., et al. (2007). Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotoxicity Research, 12, 95–104.
Racz, B., Reglodi, D., Kiss, P., et al. (2006b). In vivo neuroprotection by PACAP in excitotoxic retinal injury: review of effects on retinal morphology and apoptotic signal transduction. The International Journal of Neuroprotection and Neuroregeneration, 2, 80–85.
Racz, B., Tamas, A., Kiss, P., et al. (2006c). Involvement of ERK and CREB signalling pathways in the protective effect of PACAP on monosodium glutamate-induced retinal lesion. Annals of the New York Academy of Sciences, 1070, 507–511.
Reglodi, D., Tamas, A., Lubics, A., Szalontay, L., & Lengvari, I. (2004). Morphological and functional effects of PACAP in a 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regulatory Peptides, 123, 85–94.
Röhrenbeck, J., Wässle, H., & Heizmann, C. W. (1987). Immunocytochemical labeling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neuroscience Letters, 77, 255–260.
Sanna, P. P., Keyser, K. T., Battenberg, E., & Bloom, F. E. (1990). Parvalbumin immunoreactivity in the rat retina. Neuroscience Letters, 118, 136–139.
Seki, T., Izumi, S., Shioda, S., & Arimura, A. (2003). Pituitary adenylate cyclase activating polypeptide (PACAP) protects ganglion cell death against cutting of optic nerve in the rat retina. Regulatory Peptides, 115, 55.
Seki, T., Izumi, S., Shioda, S., Zhou, C. J., Arimura, A., & Koide, R. (2000a). Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Annals of the New York Academy of Sciences, 921, 366–369.
Seki, T., Shioda, S., Izumi, S., Arimura, A., & Koide, R. (2000b). Electron microscopic observation of pituitary adenylate cyclase activating polypeptide (PACAP)-containing neurons in the rat retina. Peptides, 21, 109–113.
Shioda, S., Ohtaki, H., Nakamachi, T., et al. (2006). Pleiotropic functions of PACAP in the CNS. Neuroprotection and neurodevelopment. Annals of the New York Academy of Sciences, 1070, 550–560.
Shoge, K., Mishima, H. K., Saitoh, T., et al. (1999). Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Research, 839, 66–73.
Somogyvari-Vigh, A., & Reglodi, D. (2004). Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Current Pharmaceutical Design, 10, 2861–2889.
Staines, D. R. (2008). Does autoimmunity of endogenous vasoactive neuropeptides cause retinopathy in humans? Medical Hypotheses, 70, 137–140.
Takamori, S., Rhee, J. S., Rosenmund, C., & Jahn, R. (2000). Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature, 407, 189–194.
Takei, N., Skoglosa, Y., & Lindholm, D. (1998). Neurotrophic and neuroprotective effects of pituitary adenylate cyclase activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. Journal of Neuroscience Research, 54, 698–706.
Tamas, A., Gabriel, R., Racz, B., et al. (2004). Effects of pituitary adenylate cyclase activating polypeptide in retinal degeneration induced by monosodium-glutamate. Neuroscience Letters, 372, 110–113.
Thoreson, W. B., & Witkovsky, P. (1999). Glutamate receptors and circuits in the vertebrate retina. Progress in Retinal and Eye Research, 18, 765–810.
Tkatchenko, A. V., Walsh, P. A., Tkatchenko, T. V., Gustincich, S., & Raviola, E. (2006). Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proceedings of the National Academy of Sciences of the United States of America, 103, 4681–4686.
Vaney, D. I. (1990). Mosaics of amacrine cells in the mammalian retina. Progress in Retinal Research, 9, 49–100.
Vereczki, V., Koves, K., Csaki, A., Grosz, K., Hoffman, G. E., & Fiskum, G. (2006). Distribution of hypothalamic, hippocampal and other limbic peptidergic neuronal cell bodies giving rise to retinopetal fibers: anterograde and retrograde tracing and neuropeptide immunohistochemical studies. Neuroscience, 140, 1089–1100.
Vugler, A. A., Redgrave, P., Semo, M., Lawrence, J., Greeanwood, J., & Coffey, P. J. (2007). Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Experimental Neurology, 205, 26–35.
Waschek, J. A. (2002). Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Developmental Neuroscience, 24, 14–23.
Wässle, H., Grunert, U., & Rohrenbeck, J. (1993). Immunocytochemical satining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. Journal of Comparative Neurology, 322, 407–421.
Witkovsky, P., & Dearry, A. (1991). Functional roles of dopamine in the vertebrate retina. Progress in Retinal Research, 11, 247–292.
Acknowledgments
This work was supported by OTKA K61766, K72592, F67830, T046589, Richter Gedeon Ltd, and ETT439/2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atlasz, T., Szabadfi, K., Kiss, P. et al. PACAP-Mediated Neuroprotection of Neurochemically Identified Cell Types in MSG-Induced Retinal Degeneration. J Mol Neurosci 36, 97–104 (2008). https://doi.org/10.1007/s12031-008-9059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9059-5