Abstract
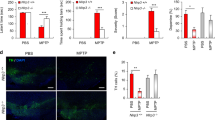
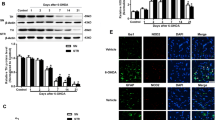
In Parkinson’s disease (PD), there is a progressive loss of neuromelanin (NM)-containing dopamine neurons in substantia nigra (SN) which is associated with microgliosis and presence of extracellular NM. Herein, we have investigated the interplay between microglia and human NM on the degeneration of SN dopaminergic neurons. Although NM particles are phagocytized and degraded by microglia within minutes in vitro, extracellular NM particles induce microglial activation and ensuing production of superoxide, nitric oxide, hydrogen peroxide (H2O2), and pro-inflammatory factors. Furthermore, NM produces, in a microglia-depended manner, neurodegeneration in primary ventral midbrain cultures. Neurodegeneration was effectively attenuated with microglia derived from mice deficient in macrophage antigen complex-1, a microglial integrin receptor involved in the initiation of phagocytosis. Neuronal loss was also attenuated with microglia derived from mice deficient in phagocytic oxidase, a subunit of NADPH oxidase, that is responsible for superoxide and H2O2 production, or apocynin, an NADPH oxidase inhibitor. In vivo, NM injected into rat SN produces microgliosis and a loss of tyrosine hydroxylase neurons. Thus, these results show that extracellular NM can activate microglia, which in turn may induce dopaminergic neurodegeneration in PD. Our study may have far-reaching implications, both pathogenic and therapeutic.




Similar content being viewed by others
Abbreviations
- COX2:
-
Cyclooxygenase 2
- DA:
-
Dopamine
- DCF:
-
Dichlorofluorescein
- GABA:
-
γ-Aminobutyric acid
- GFAP:
-
Glial filrillary acidic protein
- H2O2 :
-
Hydrogen peroxide
- IL:
-
Interleukin
- iNOS:
-
Inducible NO synthase
- iROS:
-
Intracellular ROS
- Mac-1:
-
Macrophage antigen complex-1
- Mac-1+/+ :
-
Wild-type Mac-1 mice
- Mac-1−/− :
-
Knockout Mac-1 mice
- MIP-1α:
-
Macrophage inflammatory protein-1α
- NM:
-
Neuromelanin
- NO:
-
Nitric oxide
- PD:
-
Parkinson’s disease
- PHOX:
-
Phagocytic oxidase
- PHOX+/+ :
-
PHOX wild-type mice
- PHOX−/− :
-
PHOX-deficient mice
- PMA:
-
Phorbol ester myristate
- ROS:
-
Reactive oxygen species
- SN:
-
Substantia nigra
- TH:
-
Tyrosine hydroxylase
- TH-ir:
-
TH immunoreactive
- TNF-α:
-
Tumor necrosis factor-α
References
Banati RB, Daniel SE, Blunt SB (1998) Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov Disord 13:221–227
Berger M, Budhu S, Lu E, Li Y, Loike D, Silverstein SC, Loike JD (2002) Different G(i)-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol 71:798–806
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A (2003) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 60:1059–1064
Cubells JF, Rayport S, Rajendran G, Sulzer D (1994) Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci 14:2260–2271
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Fahn S (2003) Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci 991:1–14
Farkas E, De Jong GI, de Vos RA, Jansen Steur EN, Luiten PG (2000) Pathological features of cerebral cortical capillaries are doubled in Alzheimer’s disease and Parkinson’s disease. Acta Neuropathol 100:395–402
Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem 81:1285–1297
Hattori N, Sato S (2007) Animal models of Parkinson’s disease: similarities and differences between the disease and models. Neuropathology 27:479–483
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334:345–348
Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20:6309–6316
Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46:598–605
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5:1403–1409
Liu J, He YY, Chignell CF, Clark J, Myers P, Saavedra JE, Waalkes MP (2005) Limited protective role of V-PYRRO/NO against cholestasis produced by alpha-naphthylisothiocyanate in mice. Biochem Pharmacol 70:144–151
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Pothos EN, Davila V, Sulzer D (1998) Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci 18:4106–4118
Shimohama S, Sawada H, Kitamura Y, Taniguchi T (2003) Disease model: Parkinson’s disease. Trends Mol Med 9:360–365
Staal RGW, Rayport S, Sulzer D (2006) Amperometric detection of dopamine exocytosis from synaptic terminals. In: Michael AC (ed) Electrochemical methods in neuroscience. In the series: Nicolelis M, Simon SA (eds) Methods and new frontiers In neuroscience. CRC Press, NY
Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci USA 97:11869–11874
Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L (2008) Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem 106:24–36
Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S (2003) D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901
Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R (2003) Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J 17:500–502
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22:1763–1771
Zecca L, Pietra R, Goj C, Mecacci C, Radice D, Sabbioni E (1994) Iron and other metals in neuromelanin, substantia nigra, and putamen of human brain. J Neurochem 62:1097–1101
Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D (2002) The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett 510:216–220
Zecca L, Zucca FA, Wilms H, Sulzer D (2003) Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci 26:578–580
Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG, Karatekin E, Kleinman MH, Turro N, Hornykiewicz O, Zucca FA (2004) The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci USA 101:9843–9848
Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R (2008) Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol 116:47–55
Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19:533–542
Acknowledgments
The authors thank the Legal Medicine Section, Department of Human Morphology and Biomedical Sciences, University of Milano, and the authors also thank Ms. Chiara Bellei for skilful assistance. K.P., A.A., F.A.Z., R.F., D.S., and L.Z. were supported with the grant of Michael J. Fox Foundation (New York, NY, USA), MIUR-FIRB Project RBNE03PX83_002 on Protein Folding and Aggregation: Metal and Biomolecules in Protein Conformational Diseases (Italy) and Joint Research Grant of National Parkinson Foundation-Parkinson Disease’s Foundation (Miami, FL; and New York, NY, USA). S.P. is supported by the NINDS Grants NS062180, NS11766-27A1, and NS38370-09, the NIA Grant AG21617-01A1, the US DoD Contract DAMD 17-03-1-02, as well as the Parkinson’s Disease Foundation (NY, USA), the MDA/WOW, and the Hartman Foundation. This research was supported in part by the Intramural Research Program of the NIH/NIEHS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Zhang and Kester Phillips have equally contributed to this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
NM, microglia, expression of pro-inflammatory cytokines and reactive nitrogen species. NM in microglia enriched cultures induced gene expression of mRNA of TNF-α (a; mean ± SEM; n = 3; * P < 0.001), IL-1β (b; mean ± SEM; n = 3; * P < 0.05; ** P < 0.01; *** P < 0.001) and iNOS (c; mean ± SEM; n = 3; * P < 0.01; ** P < 0.001) in a dose dependent manner (TIFF 270 kb)
Supplementary Fig. 2
NM activates microglia causing the release of NO. NM in microglia-enriched cultures induced a dose dependent release of NO measured as nitrite concentration (mean ± SEM; n = 3; * P < 0.01) (TIFF 86 kb)
Supplementary Fig. 3
NM, microglia, expression of inflammatory molecules and ROS. NM in microglia enriched cultures induced a dose dependent gene expression of MIP-1α (a; mean ± SEM; n = 3; * P < 0.05; ** P < 0.001), COX2 (b; mean ± SEM; n = 3; * P < 0.01; ** P < 0.001) and gp91 (c; mean ± SEM; n = 3; * P < 0.01; ** P < 0.001) (TIFF 274 kb)
Supplementary Fig. 4
Dose dependent release of iROS from microglia activated by NM and blockade by cytochalasin D. DCF fluorescence in response to NM, as an indicator of iROS production. (a) Indicates the dose dependence of iROS to increasing levels of NM (mean ± SEM; n = 4; * P < 0.05; ** P < 0.001). (b) iROS is inhibited by the phagocytic inhibitor, cytochalasin D (mean ± SEM; n = 3; * P < 0.05; ** P < 0.01) (TIFF 208 kb)
Rights and permissions
About this article
Cite this article
Zhang, W., Phillips, K., Wielgus, A.R. et al. Neuromelanin Activates Microglia and Induces Degeneration of Dopaminergic Neurons: Implications for Progression of Parkinson’s Disease. Neurotox Res 19, 63–72 (2011). https://doi.org/10.1007/s12640-009-9140-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9140-z