Abstract
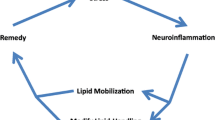
Tangles are a major histopathological feature of Alzheimer's disease and their regional location and number correlate significantly with the individual's cognitive decline. Intriguingly, these tangles are formed only in a small subset of nerve cell types and are practically absent in most animal species examined so far. In humans, tangle formation seemingly starts decades before clinical signs of dementia are seen and spread over cortical areas in a regular manner described by the Braak classification. In the present article the role of plasticity-related molecules and mechanisms are discussed considering their putative role in neuronal vulnerability and spread of tangles. Special emphasis is given to some aspects of lipid metabolism, that is, apolipoprotein E polymorphism, statin effects, and lysosomal dysfunction in Alzheimer's and Niemann-Pick C's diseases.
Similar content being viewed by others
REFERENCES
Braak, H. and Braak, E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82:239-259.
Ghebremedhin, E., Schultz, C., Thal, D. R., Rüb, U., Ohm, T. G., Braak, E., and Braak, H. 2001. Gender and age modify the association between ApoE and AD-related neuropathology. Neurology 56:1696-1701.
Ohm, T. G., Müller, H., Braak, H., and Bohl, J. 1995. Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer's disease-related neurofibrillary changes. Neuroscience 64:209-217.
Bancher, C., Braak, H., Fischer, P., and Jellinger, K. A. 1993. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease patients. Neurosci. Lett. 162:179-182.
Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T., and Hyman, B. T. 1992. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42:631-639.
Richard, S., Brion, J. P., Couck, A. M., and Flament-Durand, J. 1989. Accumulation of smooth endoplasmic reticulum in Alzheimers disease: New morphological evidence of axoplasmic flow disturbances. J. Submicrosc. Cytol. Pathol. 21:461-467.
Bobinski, M., Wegiel, J., Tarnawski, M., de Leon, M. J., Reisberg, B., Miller, D. C., and Wisniewski, H. M. 1998. Duration of neurofibrillary changes in the hippocampal pyramidal neurons. Brain Res. 799:156-158.
Morsch, R., Simon, W., and Coleman, P. D. 1999. Neurons may live for decades with neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 58:188-197.
Braak, H., Braak, E., and Kalus, P. 1989. Alzheimer's disease: Areal and laminar pathology in the occipital isocortex. Acta Neuropathol. (Berl.) 77:494-506.
Braak, H. and Braak, E. 1990. Neurofibrillary changes confined to the entorhinal region and an abundance of cortical amyloid in cases of presenile and senile dementia. Acta Neuropathol. (Berl.) 80:479-486.
Gomez Isla, T., Price, J. L., McKeel, D. W., Jr., Morris, J. C., Growdon, J. H., and Hyman, B. T. 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 16:4491-4500.
Schönheit, B., Zarski, R., and Ohm, T. G. 2003. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol. Aging (in press).
Braak, H., Braak, E., Bohl, J., and Lang, W. 1989. Alzheimer's disease: Amyloid plaques in the cerebellum. J. Neurol. Sci. 93:277-287.
Su, J. H., Deng, G., and Cotman, C. W. 1997. Transneuronal degeneration in the spread of Alzheimer's disease pathology: Immunohistochemical evidence for the transmission of tau hyperphosphorylation. Neurobiol. Dis. 4:365-375.
Amaral, D. G. and Witter, M. P. 1989. The 3-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 31:571-591.
Diekmann, S., Ohm, T. G., and Nitsch, R. 1996. Long-lasting transneuronal changes in rat dentate granule cell dendrites after entorhinal lesion: A combined intracellular injection and electron microscopic study. Brain Pathol. 6:205-215.
Miehe, U., Leranth, C., Ohm, T. G., and Nitsch, R. 1994. Long-lasting transneuronal dendritic changes of GABAergic neurons in the monkey dentate gyrus following entorhinal cortex lesion. Neurosci. Lett. 168:115-118.
Ohm, T. G., Münch, S., Schönheit, B., and Nitsch, R. 2002. Transneuronally altered dendritic processing of tangle-free neurons in Alzheimer's disease. Acta Neuropathol. (Berl.) 103:437-443.
Kohara, K., Kitamura, A., Morishima, M., and Tsumoto, T. 2001. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291:2419-2423.
Goedert, M., Spillantini, M. G., Potier, M. C., Ulrich, J., and Crowther, R. 1989. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: Differential expression of tau protein mRNAs in human brain. EMBO J. 8:393-399.
Bu, B., Klunemann, H., Suzuku, K., Li, J., Bird, T., and Jin, L.-W., and Vincent, I. 2002. Niemann-Pick disease type C yields possible clue for why cerebellar neurons do not form neurofibrillary tangles. Neurobiol. Dis. 11:285-297.
Götz, J. 2001. Tau and transgenic animal models. Brain Res. Rev. 35:266-286.
Vanier, M. T. and Suzuki, K. 1998. Recent advances in elucidating Niemann-Pick C disease. Brain Pathol. 8:163-174.
Love, S., Bridges, L. R., and Case, C. P. 1995. Neurofibrillary tangles in Niemann-Pick disease type C. Brain 118:119-129.
Horoupian, D. S. and Yang, S. S. 1978. Paired helical filaments in neurovisceral lipidosis (juvenile dystonic lipidosis). Ann. Neurol. 4:404-411.
Auer, I. A., Schmidt, M. L., Lee, V. M., Curry, B., Suzuki, K., Shin, R. W., Pentchev, P. G., Carstea, E. D., and Trojanowski, J. Q. 1995. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer's disease. Acta Neuropathol. (Berl.) 90:547-551.
Suzuki, K., Parker, C. C., Pentchev, P. G., Katz, D., Ghetti, B., D'Agostino, A., and Carstea, E. D. 1995. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol. (Berl.) 89:227-238.
Distl, R., Treiber-Held, S., Albert, F., Meske, V., Harzer, K., and Ohm, T. G. 2002. Cholesterol storage and tau-pathology in Niemann-Pick type C disease brain. J. Pathol. 200:704-777.
Carstea, E. D., Morris, J. A., Coleman, K. G., Loftus, S. K., Zhang, D., Cummings, C., Gu, J., Rosenfeld, M. A., Pavan, W. J., Krizman, D. B., Nagle, J., Polymeropoulos, M. H., Sturley, S. L., Ioannou, Y. A., Higgins, M. E., Comley, M., Cooney, A., Brown, A., Kaneski, C. R., Blanchette-Mackie, J., Dwyer, N. K., Neufeld, E. B., Chang, T.-Y., Liscum, L., Strauss III, J. F., Ohno, K., Zeigler, M., Carmi, R., Sokol, J., Markie, D., O'Neill, R. R., van Diggelen, O. P., Elleder, M., Patterson, M. C., Bradie, R. O., Vanier, M. T., Pentchev, P. G., and Tagle, D. A. 1997. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 277:288-231.
Naureckiene, S., Sleat, D. E., Lackland, H., Fensom, A., Vanier, M. T., Wattiaux, R., Jadot, M., and Lobel, P. 2000. Identification of HE1 as the second gene of Niemann-Pick C disease. Science 290:2298-2301.
Lange, Y., Ye, J., and Steck, T. L. 1998. Circulation of cholesterol between lysosomes and the plasma membrane. J. Biol. Chem. 273:18915-18922.
Neufeld, E. B., Wastney, M., Patel, S., Suresh, S., Cooney, A. M., Dwyer, N. K., Roff, C. F., Ohno, K., Morris, J. A., Carstea, E. D., Incardona, J. P., Strauss, J. F. III., Vanier, M. T., Patterson, M. C., Brady, R. O., Pentchev, P. G., and Blanchette, M.-E. 1999. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 274:9627-9635.
Zervas, M., Dobrenis, K., and Walkley, S. U. 2001. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J. Neuropathol. Exp. Neurol. 60:49-64.
Distl, R., Meske, V., and Ohm, T. G. 2001. Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol. (Berl.) 101:547-554.
Ohm, T. G., Treiber-Held, S., Distl, R., Glöckner, F., Schönheit, B., and Tamannai, M., and Meske, V. 2003. Cholesterol and tau-protein: Findings in Alzheimer's and Niemann Pick C's disease. Pharmacopsychiatry (In press).
Karten, B., Vance, D. E., Campenot, R. B., and and Vance, J. E. 2002. Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons. J. Neurochem. 83:1154-1163.
Xie, C., Burns, D. K., Turley, S. D., and Dietschy, J. M. 2000. Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J. Neuropathol. Exp. Neurol. 59:1106-1117.
German, D. C., Quintero, E. M., Liang, C. L., Ng, B., Punia, S., Xie, C., and Dietschy, J. M. 2001. Selective neurodegeneration, without neurofibrillary tangles, in a mouse model of Niemann-Pick C disease. J. Comp. Neurol. 433:415-425.
Sawamura, N., Gong, J. S., Garver, W. S., Heidenreich, R. A., Ninomiya, H., Ohno, K., Yanagisawa, K., and Michikawa, M. 2001. Site-specific phosphorylation of tau accompanied by activation of mitogen-activated protein kinase (MAPK) in brains of Niemann-Pick type C mice. J. Biol. Chem. 276:10314-10319.
Bu, B., Li, J., Davies, P., and Vincent, I. 2002. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J. Neurosci. 22:6515-6525.
Alonso, A., Zaidi, T., Novak, M., Grundke-Iqbal, I., and Iqbal, K. 2001. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. 98:6923-6928.
Cedazo-Minguez, A., Hamker, U., Veh, R., Meske, V., Albert, F., Cowburn, R. F., and Ohm, T. G. 2001. Regulation of apolipoprotein E secretion in rat primary hippocampal astrocyte cultures. Neuroscience 105:651-661.
Genis, L., Chen, Y., Shohami, E., and Michaelson, D. M. 2000. Tau hyperphosphorylation in apolipoprotein E-deficient and control mice after closed head injury. J. Neurosci. Res. 60:559-564.
Bi, X., Yong, A. P., Zhou, J., Ribak, C. E., and Lynch, G. 2001. Rapid induction of intraneuronal neurofibrillary tangles in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. 98:8832-8837.
Saunders, A. M., Strittmatter, W. J., Schmechel, D., St. George-Hyslop, P. H., Pericak Vance, M. A., Joo, S. H., Rosi, B. L., Gusella, J. F., Crapper-MacLachlan, D. R., Alberts, M. J., Hulette, C., Crain, B., Goldgaber, D., and Roses, A. D. 1993. Association of apolipoprotein E allele epsilon-4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43:1467-1472.
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Rimmler, J. B., Locke, P. A., Conneally, P. M., Schmader, K. E., Tanzi, R. E., Gusella, J. F., Small, G. W., Roses, A. D., Pericak Vance, M. A., and Haines, J. L. 1995. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology 45:1323-1328.
Ohm, T. G., Kirca, M., Bohl, J., Scharnagl, H., Gross, W., and März, W. 1995. Apolipoprotein E polymorphism influences not only cerebral senile plaque load but also Alzheimer-type neurofibrillary tangle formation. Neuroscience 66:583-587.
Ohm, T. G., Scharnagl, H., März, W., and Bohl, J. 1999. Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer's disease-related lesions. Acta Neuropathol. (Berl.) 98:273-280.
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, Jr., Rimmler, J. B., Locke, P. A., Conneally, P. M., Schmader, K. E., Tanzi, R. E., and et al. 1995. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology 45:1323-1328.
Poirier, J., May, P. C., Osterburg, H. H., Geddes, J., Cotman, C. W., and Finch, C. E. 1990. Selective alterations of RNA in rat hippocampus after entorhinal cortex lesioning. Proc. Nat. Acad. Sci. 87:303-307.
Poirier, J. 1994. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 17:525-530.
Poirier, J., Baccichet, A., Dea, D., and Gauthier, S. 1993. Cholesterol synthesis and lipoprotein reuptake during synaptic remodelling in hippocampus in adult rats. Neuroscience 55:81-90.
Petegnief, V., Saura, J., Gregorio-Rocasolano, N., and Paul, S. M. 2001. Neuronal injury-induced expression and release of apolipoprotein E in mixed neuron/glia co-cultures: Nuclear factor kappaB inhibitors reduce basal and lesion-induced secretion of apolipoprotein E. Neuroscience 104:223-234.
Arendt, T., Brückner, M. K., Gertz, H. J., and Marcova, L. 1998. Cortical distribution of neurofibrillary tangles in Alzheimer's disease matches the pattern of neurons that retain their capacity of plastic remodelling in the adult brain. Neuroscience 83:991-1002.
Arendt, T., Schindler, C., Brückner, M. K., Eschrich, K., Bigl, V., Zedlick, D., and Markova, L. 1997. Plastic neuronal remodeling is impaired in patients with Alzheimer's disease carrying apolipoprotein ε4 allele. J. Neurosci. 17:516-529.
Glöckner, F., Meske, V., and Ohm, T. G. 2002. Genotype-related differences of hippocampal apolipoprotein E levels only in early stages of neuropathological changes in Alzheimer's disease. Neuroscience 114:1103-1114.
Torack, R. M. and Miller, J. W. 1995. Denervation induced abnormal phosphorylation in hippocampal neurons. Brain Res. 669:135-139.
Nathan, B. P., Bellosta, S., Sanan, D. A., Weisgraber, K. H., Mahley, R. W., and Pitas, R. 1994. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science 264:850-852.
Nathan, B. P., Jiang, Y., Wong, G. K., Shen, F., Brewer, G. J., and Struble, R. G. 2002. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein. Brain Res. 928:96-105.
Zetterberg, H., Palmer, M., Ricksten, A., Poirier, J., Palmqvist, L., Rymo, L., Zafiropoulos, A., Arvanitis, D. A., Spandidos, D. A., and Blennow, K. 2002. Influence of the apolipoprotein E epsilon4 allele on human embryonic development. Neurosci. Lett. 324:189-192.
Yu, Y. W., Lin, C. H., Chen, S. P., Hong, C. J., and Tsai, S. J. 2000. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci. Lett. 294:179-181.
White, F., Nicoll, J. A., Roses, A. D., and Horsburgh, K. 2001. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol. Dis. 8:611-625.
Müller, W., Meske, V., Berlin, K., Scharnagl, H., März, W., and Ohm, T. G. 1998. Apolipoprotein E isoforms increase intracellular Ca2+ differentially through an omega-agatoxin IVa-sensitive Ca2+-channel. Brain Pathol. 8:641-653.
Wisniewski, T., Castaño, E. M., Golabek, A., Vogel, T., and Frangione, B. 1994. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am. J. Pathol. 145:1030-1035.
Barger, S. W. and Harmon, A. D. 1997. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 388:878-881.
Cullen, P., Cignarella, A., Brennhausen, B., Mohr, S., Assmann, G., and von Eckardstein, A. 1998. Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J. Clin. Invest. 101:1670-1677.
Igbavboa, U., Avdulov, N. A., Schroeder, F., and Wood, W. G. 1996. Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J. Neurochem. 66:1717-1725.
Göritz, C., Mauch, D. H., Nägler, K. and Pfrieger, F. W. 2002. Role of glia-derived cholesterol in synaptogenesis: New revelations in the synapse-glia affair. J. Physiol. Paris 96:257-263.
Mitter, D., Reisinger, C., Hinz, H., Hollmann, S., Yelamanchili, S. V., Treiber-Held, S., Ohm, T. G., Herrmann, A., and Ahnert-Hilger, G. 2003. The synaptophysin/synaptobrevin interaction critically depends on the cholesterol content. J. Neurochem. 84:35-42.
Meske, V., Albert, F., Richter, D., Schwarze, J., and Ohm, T. G. 2003. Blockade of HMG-CoA reductase activity causes changes in microtubule-stabilizing protein tau via suppression of geranyl-geranylpyrophosphate formation: Implications for Alzheimer's disease. Eur. J. Neurosci. 17:93-102.
Dietschy, J. M. and Turley, S. D. 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12:105-112.
Braak, H. and Braak, E. 1996. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. (Berl.) 92:197-201.
Fagan, A. M., Murphy, B. A., Patel, S. N., Kilbridge, J. F., Mobley, W. C., Bu, G., and Holtzman, D. M. 1998. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE (—/—) mice but impaired clearance of axonal degeneration products following injury. Exp. Neurol. 151:314-325.
Gamblin, T. C., King, M. E., Koret, J., Berry, R. W., and Binder, L. I. 2001. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry 39:14203-14210.
Davies, J. P. 2000. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science 290:2295-2298.
Cataldo, A. M., Barnett, J. L., Berman, S. A., Li, J., Quarless, S., Bursztajn, S., Lippa, C., and Nixon, R. A. 1995. Gene expression and cellular content of cathepsin D in Alzheimer's disease brain: Evidence for early up-regulation of the endosomal-lysosomal system. Neuron 14:671-680.
Bednarski, E. and Lynch, G. 1996. Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J. Neurochem. 67:1846-1855.
Bednarski, E. and Lynch, G. 1998. Selective suppression of cathepsin L results from elevations in lysosomal pH and is followed by proteolysis of tau protein. Neuroreport 9:2089-2094.
Bi, X., Zhou, J., and Lynch, G. 1999. Lysosomal protease inhibitors induce meganeurites and tangle-like structures in entorhinohippocampal regions vulnerable to Alzheimer's disease. Exp. Neurol. 158:312-327.
Yamazaki, T., Chang, T. Y., Haass, C., and Ihara, Y. 2001. Accumulation and aggregation of amyloid beta-protein in late endosomes of Niemann-pick type C cells. J. Biol. Chem. 276:4454-4460.
Scharnagl, H., Tisljar, U., Winkler, K., Hüttinger, M., Nauck, M. A., Gross, W., Wieland, H., Ohm, T. G., and März, W. 1999. The A4 amyloid peptide enhances the uptake of beta-very low density lipoproteins by the low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor and heparan sulfate proteoglycans pathway. Lab. Invest. 79:1271-1286.
Winkler, K., Scharnagl, H., Tisljar, U., Hoschutzky, H., Friedrich, I., Hoffmann, M. M., Hüttinger, M., Wieland, H., and März, W. 1999. Competition of Abeta amyloid peptide and apolipoprotein E for receptor-mediated endocytosis. J. Lipid Res. 40:447-455.
Miklossy, J. and Van der Loos, H. 1987. Cholesterol ester crystals in polarized light show pathways in the human brain. Brain Res. 426:377-380.
Yang, A. J., Chandswangbhuvana, D., Margol, L., and Glabe, C. G. 1998. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1-42 pathogenesis. J. Neurosci. Res. 52:691-698.
Ditaranto, K., Tekirian, T. L., and Yang, A. J. 2001. Lysosomal membrane damage in soluble Abeta-mediated cell death in Alzheimer's disease. Neurobiol. Dis. 8:19-31.
Friedhoff, P., von Bergen, M., Mandelkow, E. M., Davies, P., and Mandelkow, E. 1998. A nucleated assembly mechanism of Alzheimer paired helical filaments. Proc. Natl. Acad. Sci. 95:15712-15717.
Braak, E., Braak, H., and Mandelkow, E. M. 1994. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. (Berl.) 87:554-567.
Wolozin, B., Kellman, W., Ruosseau, P., Celesia, G. G., and Siegel, G. 2000. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 57:1439-1443.
Jick, H., Zornberg, G. L., Jick, S. S., Seshadri, S., and Drachman, D. A. 2000. Statins and the risk of dementia. Lancet 356:1627-1631.
Wong, W. W., Dimitroulakos, J., Minden, M. D., and Penn, L. Z. 2002. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16:508-519.
Naidu, A., Xu, Q., Catalano, R., and Cordell, B. 2002. Secretion of apolipoprotein E by brain glia requires protein prenylation and is suppressed by statins. Brain Res. 958:100-111.
Hoyer, S. 2000. Brain glucose and energy metabolism abnormalitites in sporadic Alzheimer disease: Causes and consequences—an update. Exp. Gerontol. 35:1363-1372.
Markesbery, W. R. 1997. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 23:134-147.
Mandelkow, E. M. and Mandelkow, E. 1998. Tau in Alzheimer's disease. Trends Cell. Biol. 8:425-427.
Hartmann, T. 2001. Cholesterol, A beta and Alzheimer's disease. Trends Neurosci. 24:S45-S48.
Kureishi, Y., Luo, Z., Shiojima, I., Bialik, A., Fulton, D., Lefer, D. J., Sessa, W. C., and Walsh, K. 2000. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 6:1004-1010.
Hanger, D. P., Hughes, K., Woodgett, J. R., Brion, J. P., and Anderton, B. H. 1992. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 147:58-62.
Lovestone, S. and Reynolds, C. H. 1997. The phosphorylation of tau: A critical stage in neurodevelopment and neurodegenerative processes. Neuroscience 78:309-324.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to Dr. Carl Cotman.
Rights and permissions
About this article
Cite this article
Ohm, T.G., Glöckner, F., Distl, R. et al. Plasticity and the Spread of Alzheimer's Disease-Like Changes. Neurochem Res 28, 1715–1723 (2003). https://doi.org/10.1023/A:1026017206925
Issue Date:
DOI: https://doi.org/10.1023/A:1026017206925