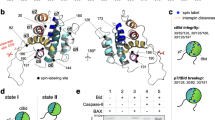
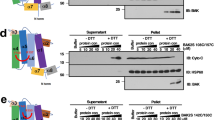
Abstract
Recent evidence supports the theory that mitochondrial homeostasis is the key regulatory step in apoptosis through the actions of members of the Bcl-2 family1,2,3. Pro-apoptotic members of the family, such as Bax, Bad and Bid, can induce the loss of outer-membrane integrity with subsequent redistribution of pro-apoptotic proteins such as cytochrome c that are normally located in the intermembrane spaces of mitochondria2. The anti-apoptotic members of the family, such as Bcl-2 and Bcl-XL, protect the integrity of the mitochondrion and prevent the release of death-inducing factors1,2,3. Bid normally exists in an inactive state in the cytosol, but after cleavage by caspase 8, the carboxy-terminal portion (tBid) moves from cytosol to mitochondria, where it induces release of cytochrome c4,5. Here we address the question of what mediates specific _targeting of tBid to the mitochondria. We provide evidence that cardiolipin, which is present in mitochondrial membranes, mediates the _targeting of tBid to mitochondria through a previously unkown three-helix domain in tBid. These findings implicate cardiolipin in the pathway for cytochrome c release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Adams, J. M. & Cory, S. Science. 281, 1322–1326 (1998).
Green, D. R. & Reed, J. C. Science. 281, 1309–1311 (1998).
Vaux, D. L., & Korsmeyer, S. J. Cell 96, 245–254 0 (1999).
Li, H. et al. Cell 94, 491–501 (1998).
Luo, X. et al. Cell 94, 481–490 (1998).
Ohtsuka, T. et al. J. Biol. Chem. 268, 22908–22913 (1993).
Kawasaki, K. et al. J. Biol. Chem. 274, 1828–1834 (1999).
Chou, J. J. et al. Cell 96, 615–624 (1999).
McDonnell, J. M. et al. Cell 96, 625–634 (1999).
Ardail, D. et al. J. Biol. Chem. 265, 18797–18802 (1990).
Cullis, P. R. & De Kruijff, B. Biochim. Biophys. Acta. 559, 399–420 (1979).
Lieser, M. et al. Gastroenterology 115, 693–701 (1998).
Tarantini, F. et al. J. Biol. Chem. 270, 29039–29042 (1995).
Li, K. et al. Cell 101, 389–399 (2000).
Acknowledgements
We thank Y. Li and R. Harold for technical assistance. M.L. is supported by an NIH MSTP training grant (5-T32-GM08014). X.W. is also supported by grants from the NIH (GMRO1-55942) and the Robert Welch Foundation (I-1412). X.X. is supported by a NIH grant (DKRO1-33627).
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence and requests for materials should be addressed to X. W.
Rights and permissions
About this article
Cite this article
Lutter, M., Fang, M., Luo, X. et al. Cardiolipin provides specificity for _targeting of tBid to mitochondria. Nat Cell Biol 2, 754–756 (2000). https://doi.org/10.1038/35036395
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35036395
This article is cited by
-
ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease
Cell Communication and Signaling (2024)
-
A BAK subdomain that binds mitochondrial lipids selectively and releases cytochrome C
Cell Death & Differentiation (2023)
-
Barth syndrome: cardiolipin, cellular pathophysiology, management, and novel therapeutic _targets
Molecular and Cellular Biochemistry (2021)
-
Crosstalk Between Lipids and Mitochondria in Diabetic Kidney Disease
Current Diabetes Reports (2019)
-
Liver-specific Bid silencing inhibits APAP-induced cell death in mice
Apoptosis (2019)