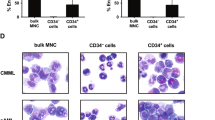
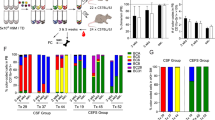
Abstract
Mixed lineage leukemia (MLL)-fusion proteins can induce acute myeloid leukemias (AMLs) from either hematopoietic stem cells (HSCs) or granulocyte–macrophage progenitors (GMPs), but it remains unclear whether the cell of origin influences the biology of the resultant leukemia. MLL-AF9-transduced single HSCs or GMPs could be continuously replated, but HSC-derived clones were more likely than GMP-derived clones to initiate AML in mice. Leukemia stem cells derived from either HSCs or GMPs had a similar immunophenotype consistent with a maturing myeloid cell (LGMP). Gene expression analyses demonstrated that LGMP inherited gene expression programs from the cell of origin including high-level Evi-1 expression in HSC-derived LGMP. The gene expression signature of LGMP derived from HSCs was enriched in poor prognosis human MLL-rearranged AML in three independent data sets. Moreover, global 5′-mC levels were elevated in HSC-derived leukemias as compared with GMP-derived leukemias. This mirrored a difference seen in 5′-mC between MLL-rearranged human leukemias that are either EVI1 positive or EVI1 negative. Finally, HSC-derived leukemias were more resistant to chemotherapy than GMP-derived leukemias. These data demonstrate that the cell of origin influences the gene expression profile, the epigenetic state and the drug response in AML, and that these differences can account for clinical heterogeneity within a molecularly defined group of leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schlenk RF, Dohner K . Impact of new prognostic markers in treatment decisions in acute myeloid leukemia. Curr Opin Hematol 2009; 16: 98–104.
Ross ME, Mahfouz R, Onciu M, Liu HC, Zhou X, Song G et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood 2004; 104: 3679–3687.
Lowenberg B . Acute myeloid leukemia: the challenge of capturing disease variety. Hematology Am Soc Hematol Educ Program 2008; 2008: 1–11.
Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med 2011; 17: 1086–1093.
Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA . Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA 2010; 304: 2706–2715.
Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med 2004; 350: 1617–1628.
Krivtsov AV, Armstrong SA . MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer 2007; 7: 823–833.
Krauter J, Wagner K, Schafer I, Marschalek R, Meyer C, Heil G et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol 2009; 27: 3000–3006.
Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood 2009; 114: 2489–2496.
Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL . Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev 2003; 17: 3029–3035.
Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006; 442: 818–822.
Schlenk RF, Dohner K, Kneba M, Gotze K, Hartmann F, Del Valle F et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica 2009; 94: 54–60.
Stubbs MC, Kim YM, Krivtsov AV, Wright RD, Feng Z, Agarwal J et al. MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment. Leukemia 2008; 22: 66–77.
Somervaille TC, Cleary ML . Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 2006; 10: 257–268.
Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z et al. The Wnt/β-catenin Pathway is required for the development of leukemia stem cells in AML. Science 2010; 327: 1650–1653.
Osawa M, Hanada K, Hamada H, Nakauchi H . Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996; 273: 242–245.
Wong P, Iwasaki M, Somervaille TC, So CW, Cleary ML . Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev 2007; 21: 2762–2774.
Arai S, Yoshimi A, Shimabe M, Ichikawa M, Nakagawa M, Imai Y et al. Evi-1 is a transcriptional _target of MLL oncoproteins in hematopoietic stem cells. Blood 2010; 117: 6304–6314.
Bindels EM, Havermans M, Lugthart S, Erpelinck C, Wocjtowicz E, Krivtsov AV et al. EVI1 is critical for the pathogenesis of a subset of MLL-AF9-rearranged AMLs. Blood 2012; 119: 5838–5849.
Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck CA, Valk PJ et al. High EVI1 levels predict adverse outcome in acute myeloid leukemia: prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood 2008; 111: 4329–4337.
Haas K, Kundi M, Sperr WR, Esterbauer H, Ludwig WD, Ratei R et al. Expression and prognostic significance of different mRNA 5'-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosomes Cancer 2008; 47: 288–298.
Groschel S, Schlenk RF, Engelmann J, Rockova V, Teleanu V, Kuhn MW et al. Deregulated expression of EVI1 defines a Poor prognostic subset of MLL-Rearranged acute myeloid leukemias: a study of the German-Austrian Acute Myeloid Leukemia Study Group and the Dutch-Belgian-Swiss HOVON/SAKK Cooperative Group. J Clin Oncol 2012; 31: 95–103.
Bruns I, Czibere A, Fischer JC, Roels F, Cadeddu RP, Buest S et al. The hematopoietic stem cell in chronic phase CML is characterized by a transcriptional profile resembling normal myeloid progenitor cells and reflecting loss of quiescence. Leukemia 2009; 23: 892–899.
Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med 2010; 16: 903–908.
Noordermeer SM, Sanders MA, Gilissen C, Tonnissen E, van der Heijden A, Dohner K et al. High BRE expression predicts favorable outcome in adult acute myeloid leukemia, in particular among MLL-AF9-positive patients. Blood 2011; 118: 5613–5621.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 2010; 17: 13–27.
Broske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet 2009; 41: 1207–1215.
Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, Khrebtukova I et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet 2012; 8: e1002781.
Trowbridge JJ, Sinha AU, Zhu N, Li M, Armstrong SA, Orkin SH . Haploinsufficiency of Dnmt1 impairs leukemia stem cell function through derepression of bivalent chromatin domains. Genes Dev 2012; 26: 344–349.
Figueroa ME, Melnick A, Greally JM . Genome-wide determination of DNA methylation by Hpa II tiny fragment enrichment by ligation-mediated PCR (HELP) for the study of acute leukemias. Methods Mol Biol 2009; 538: 395–407.
Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 2009; 360: 470–480.
Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev 2009; 23: 877–889.
Visvader JE . Cells of origin in cancer. Nature 2011; 469: 314–322.
Wang PY, Young F, Chen CY, Stevens BM, Neering SJ, Rossi RM et al. The biologic properties of leukemias arising from BCR/ABL-mediated transformation vary as a function of developmental origin and activity of the p19ARF gene. Blood 2008; 112: 4184–4192.
Chen D, Livne-bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R . Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 2004; 5: 539–551.
Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet 2009; 41: 178–186.
Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 2004; 6: 587–596.
Parekh S, Prive G, Melnick A . Therapeutic _targeting of the BCL6 oncogene for diffuse large B-cell lymphomas. Leuk Lymphoma 2008; 49: 874–882.
Selmi T, Martello A, Vignudelli T, Ferrari E, Grande A, Gemelli C et al. ZFP36 expression impairs glioblastoma cell lines viability and invasiveness by _targeting multiple signal transduction pathways. Cell Cycle 2012; 11: 1977–1987.
Bullinger L, Ehrich M, Dohner K, Schlenk RF, Dohner H, Nelson MR et al. Quantitative DNA methylation predicts survival in adult acute myeloid leukemia. Blood 2009; 115: 636–642.
Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood 2009; 114: 3448–3458.
Iliopoulos D, Hirsch HA, Struhl K . An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009; 139: 693–706.
Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 2009; 16: 379–389.
Chen J, McKay RM, Parada LF . Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 2012; 149: 36–47.
Lei L, Sonabend AM, Guarnieri P, Soderquist C, Ludwig T, Rosenfeld S et al. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One 2011; 6: e20041.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110.
Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 2010; 28: 848–855.
Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010; 467: 285–290.
Jones PA, Baylin SB . The epigenomics of cancer. Cell 2007; 128: 683–692.
Feinberg AP, Ohlsson R, Henikoff S . The epigenetic progenitor origin of human cancer. Nat Rev Genet 2006; 7: 21–33.
Acknowledgements
We thank Renee Wright for technical help and Megan Smith for administrative assistance. This work was supported in part by the National Cancer Institute (5P01CA66996), the Leukemia and Lymphoma Society, the American Cancer Society and the Harvard Stem Cell Institute, and the German Research Foundation (DFG Heisenberg-Stipendium BU 1339/3-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Krivtsov, A., Figueroa, M., Sinha, A. et al. Cell of origin determines clinically relevant subtypes of MLL-rearranged AML. Leukemia 27, 852–860 (2013). https://doi.org/10.1038/leu.2012.363
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.363