Abstract
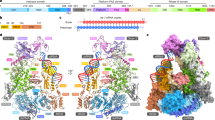
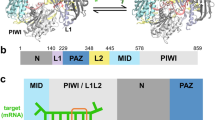
RNA interference is a conserved mechanism that regulates gene expression in response to the presence of double-stranded (ds)RNAs1,2. The RNase III-like enzyme Dicer first cleaves dsRNA into 21–23-nucleotide small interfering RNAs (siRNAs)3,4,5,6. In the effector step, the multimeric RNA-induced silencing complex (RISC) identifies messenger RNAs homologous to the siRNAs and promotes their degradation3,7. The Argonaute 2 protein (Ago2) is a critical component of RISC8,9. Both Argonaute and Dicer family proteins contain a common PAZ domain whose function is unknown10. Here we present the three-dimensional nuclear magnetic resonance structure of the Drosophila melanogaster Ago2 PAZ domain. This domain adopts a nucleic-acid-binding fold that is stabilized by conserved hydrophobic residues. The nucleic-acid-binding patch is located in a cleft between the surface of a central β-barrel and a conserved module comprising strands β3, β4 and helix α3. Because critical structural residues and the binding surface are conserved, we suggest that PAZ domains in all members of the Argonaute and Dicer families adopt a similar fold with nucleic-acid binding function, and that this plays an important part in gene silencing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Denli, A. M. & Hannon, G. J. RNAi: an ever-growing puzzle. Trends Biochem. Sci. 28, 196–201 (2003)
Hutvagner, G. & Zamore, P. D. RNAi: nature abhors a double-strand. Curr. Opin. Genet. Dev. 12, 225–232 (2002)
Hammond, S. M., Bernstein, E., Beach, D. & Hannon, G. J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296 (2000)
Zamore, P. D., Tuschl, T., Sharp, P. A. & Bartel, D. P. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101, 25–33 (2000)
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001)
Elbashir, S. M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001)
Nykanen, A., Haley, B. & Zamore, P. D. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell 107, 309–321 (2001)
Hammond, S. M., Boettcher, S., Caudy, A. A., Kobayashi, R. & Hannon, G. J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293, 1146–1150 (2001)
Martinez, J., Patkaniowska, A., Urlaub, H., Luhrmann, R. & Tuschl, T. Single-stranded antisense siRNAs guide _target RNA cleavage in RNAi. Cell 110, 563–574 (2002)
Cerutti, L., Mian, N. & Bateman, A. Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain. Trends Biochem. Sci. 25, 481–482 (2000)
Kataoka, Y., Takeichi, M. & Uemura, T. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells 6, 313–325 (2001)
Carmell, M. A., Xuan, Z., Zhang, M. Q. & Hannon, G. J. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 16, 2733–2742 (2002)
Tabara, H. et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99, 123–132 (1999)
Theobald, D. L., Mitton-Fry, R. M. & Wuttke, D. S. Nucleic acid recognition by OB-fold proteins. Annu. Rev. Biophys. Biomol. Struct. 32, 115–133 (2003)
Volpe, T. A. et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002)
Yan, K. S. et al. Structure and conserved RNA binding of the PAZ domain. Nature advance online publication, 16 November 2003 (doi:10.1038/nature02129)
Zhang, H., Kolb, F. A., Brondani, V., Billy, E. & Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21, 5875–5885 (2002)
Elbashir, S. M., Martinez, J., Patkaniowska, A., Lendeckel, W. & Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20, 6877–6888 (2001)
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX Pipes. J. Biomol. NMR 6, 277–293 (1995)
Johnson, B. A. & Blevins, R. A. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 (1994)
Sattler, M., Schleucher, J. & Griesinger, C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. NMR Spectrosc. 34, 93–158 (1999)
Cornilescu, G., Delaglio, F. & Bax, A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13, 289–302 (1999)
Linge, J. P., O'Donoghue, S. I. & Nilges, M. Automated assignment of ambiguous nuclear overhauser effects with ARIA. Methods Enzymol. 339, 71–90 (2001)
Brünger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Linge, J. P., Williams, M. A., Spronk, C. A., Bonvin, A. M. & Nilges, M. Refinement of protein structures in explicit solvent. Proteins 50, 496–506 (2003)
Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R. & Thornton, J. M. AQUA ROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 (1996)
Koradi, R., Billeter, M. & Wüthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 (1996)
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Protein Struct. Funct. Genet. 11, 281–296 (1991)
Grüter, P. et al. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1, 649–659 (1998)
Bogden, C. E., Fass, D., Bergman, N., Nichols, M. D. & Berger, J. M. The structural basis for terminator recognition by the Rho transcription termination factor. Mol. Cell 3, 487–493 (1999)
Acknowledgements
We thank A. Ladurner, E. Conti and R. Russell for discussions; G. Stier and S. Bäckström for the pETM60 vector; M. Rode for technical support; F. Ciccarreli and P. Bork for help with sequence alignments; and the NMR centre in Frankfurt, Germany for NMR measurement time. This study was supported by the European Molecular Biology Organization (EMBO), the German Research Foundation (DFG), and the Human Frontier Science Program Organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Lingel, A., Simon, B., Izaurralde, E. et al. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature 426, 465–469 (2003). https://doi.org/10.1038/nature02123
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature02123