Abstract
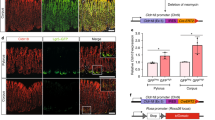
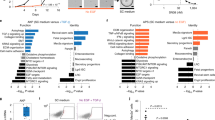
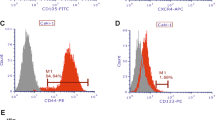
Colon cancer is one of the best-understood neoplasms from a genetic perspective1,2,3, yet it remains the second most common cause of cancer-related death, indicating that some of its cancer cells are not eradicated by current therapies4,5. What has yet to be established is whether every colon cancer cell possesses the potential to initiate and sustain tumour growth, or whether the tumour is hierarchically organized so that only a subset of cells—cancer stem cells—possess such potential6,7. Here we use renal capsule transplantation in immunodeficient NOD/SCID mice to identify a human colon cancer-initiating cell (CC-IC). Purification experiments established that all CC-ICs were CD133+; the CD133- cells that comprised the majority of the tumour were unable to initiate tumour growth. We calculated by limiting dilution analysis that there was one CC-IC in 5.7 × 104 unfractionated tumour cells, whereas there was one CC-IC in 262 CD133+ cells, representing >200-fold enrichment. CC-ICs within the CD133+ population were able to maintain themselves as well as differentiate and re-establish tumour heterogeneity upon serial transplantation. The identification of colon cancer stem cells that are distinct from the bulk tumour cells provides strong support for the hierarchical organization of human colon cancer, and their existence suggests that for therapeutic strategies to be effective, they must _target the cancer stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal cancer tumorigenesis. Cell 61, 759–767 (1990)
Fearon, E. R. & Jones, P. A. Progressing toward a molecular description of colorectal cancer development. FASEB J. 6, 2783–2790 (1992)
Radtke, F. & Clevers, H. Self-renewal and cancer of the gut: two sides of a coin. Science 307, 1904–1909 (2005)
Nelson, H. et al. Guidelines 2000 for colon and rectal cancer surgery. J. Natl Cancer Inst. 93, 583–596 (2001)
O'Connell, J. B., Maggard, M. A. & Ko, C. Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J. Natl Cancer Inst. 96, 1420–1425 (2004)
Dick, J. E. Breast cancer stem cells revealed. Proc. Natl Acad. Sci. USA 100, 3547–3549 (2003)
Wang, J. C. & Dick, J. E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005)
Pocard, M., Tsukui, H., Salmon, R. J., Dutrillaux, B. & Poupon, M. F. Efficiency of orthotopic xenograft models for human colon cancers. In Vivo 10, 463–469 (1996)
Ravi, R. et al. Elimination of hepatic metastases of colon cancer cells via p53-independent cross-talk between irinotecan and Apo2 ligand/TRAIL. Cancer Res. 64, 9105–9114 (2004)
Golas, J. M. et al. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 65, 5358–5364 (2005)
Sack, M. J. & Roberts, S. A. Cytokeratins 20 and 7 in the differential diagnosis of metastatic carcinoma in cytologic specimens. Diagn. Cytopathol. 16, 132–136 (1997)
Ishida, H. et al. Ki-67 and CEA expression as prognostic markers in Dukes' C colorectal cancer. Cancer Lett. 207, 109–115 (2004)
Liang, J. T. et al. Microvessel density, cyclo-oxygenase 2 expression, K-ras mutation and p53 overexpression in colonic cancer. Br. J. Surg. 91, 355–361 (2004)
Porter, E. H. & Berry, R. J. The efficient design of transplantable tumour assays. Br. J. Cancer 17, 583–595 (1964)
Wang, J. C., Doedens, M. & Dick, J. E. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood 89, 3919–3924 (1997)
Al Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003)
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004)
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001)
Marzesco, A. M. et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci. 118, 2849–2858 (2005)
Corbeil, D. et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and _targeted to plasma membrane protrusions. J. Biol. Chem. 275, 5512–5520 (2000)
Madlambayan, G. J. et al. Dynamic changes in cellular and microenvironmental composition can be controlled to elicit in vitro human hematopoietic stem cell expansion. Exp. Hematol. 33, 1229–1239 (2005)
Lapidot, T. et al. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature 367, 645–648 (1994)
Hope, K. J., Jin, L. & Dick, J. E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol. 5, 738–743 (2004)
Al Hajj, M. & Clarke, M. F. Self-renewal and solid tumor stem cells. Oncogene 23, 7274–7282 (2004)
Polyak, K. & Hahn, W. C. Roots and stems: stem cells in cancer. Nature Med. 12, 296–300 (2006)
Al Hajj, M., Becker, M. W., Wicha, M., Weissman, I. & Clarke, M. F. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 14, 43–47 (2004)
Acknowledgements
We gratefully acknowledge assistance from F. Meng, H. Begley and C. Ash for tissue acquisition, D. Hedley for advice on establishment of the xenograft model, J Wang for assistance with manuscript preparation, and the Dick laboratory members, P. Dirks and D.Hill for comments on the manuscript. We also acknowledge K. So and the University Health Network Pathology Research Program for tissue sectioning and immunohistochemistry. This work was supported by: a clinician-scientist award (C.A.O’B.), and grants (J.E.D.) from the Canadian Institute of Health Research, as well as grants to J.E.D. from Genome Canada through the Ontario Genomics Institute, the Ontario Cancer Research Network with funds from the Province of Ontario, the Leukemia and Lymphoma Society, the National Cancer Institute of Canada with funds from the Canadian Cancer Society and the Terry Fox Foundation, and a Canada Research Chair (J.E.D.). Author Contributions C.A.O'B. planned the project, carried out experimental work, analysed data and prepared the manuscript. A.P. provided pathology analysis. S.G. provided clinical information and human tissues. J.E.D. planned the project, analysed data, and prepared the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–4. Supplementary Figure 1: Unfractionated and CD133+ colon cancer cells initiate tumors when transplanted under the renal capsule of NOD/SCID mice. Supplementary Figure 2: Flow cytometric analysis of CD133 and ESA expression. Supplementary Figure 3: Histological examination following injection of CD133- colon cancer cells. Supplementary Figure 4: Analysis of purity following magnetic bead separation (PDF 1021 kb)
Supplementary Methods
This file contains additional details on the methods used in this study. (DOC 28 kb)
Rights and permissions
About this article
Cite this article
O’Brien, C., Pollett, A., Gallinger, S. et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007). https://doi.org/10.1038/nature05372
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05372
This article is cited by
-
Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease
Cellular & Molecular Biology Letters (2024)
-
Repurposing the dopamine transporter antagonist vanoxerine to treat colorectal cancer
Nature Cancer (2024)
-
Membrane to cortex attachment determines different mechanical phenotypes in LGR5+ and LGR5- colorectal cancer cells
Nature Communications (2024)
-
Clonal tracking in cancer and metastasis
Cancer and Metastasis Reviews (2024)
-
_targeting cancer stem cell OXPHOS with tailored ruthenium complexes as a new anti-cancer strategy
Journal of Experimental & Clinical Cancer Research (2024)