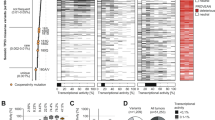
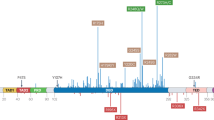
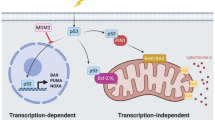
Abstract
Tumorigenesis is a multi-step process that requires activation of oncogenes and inactivation of tumour suppressor genes1. Mouse models of human cancers have recently demonstrated that continuous expression of a dominantly acting oncogene (for example, Hras, Kras and Myc) is often required for tumour maintenance2,3,4,5; this phenotype is referred to as oncogene addiction6. This concept has received clinical validation by the development of active anticancer drugs that specifically inhibit the function of oncoproteins such as BCR-ABL, c-KIT and EGFR7,8,9,10. Identifying additional gene mutations that are required for tumour maintenance may therefore yield clinically useful _targets for new cancer therapies. Although loss of p53 function is a common feature of human cancers11, it is not known whether sustained inactivation of this or other tumour suppressor pathways is required for tumour maintenance. To explore this issue, we developed a Cre-loxP-based strategy to temporally control tumour suppressor gene expression in vivo. Here we show that restoring endogenous p53 expression leads to regression of autochthonous lymphomas and sarcomas in mice without affecting normal tissues. The mechanism responsible for tumour regression is dependent on the tumour type, with the main consequence of p53 restoration being apoptosis in lymphomas and suppression of cell growth with features of cellular senescence in sarcomas. These results support efforts to treat human cancers by way of pharmacological reactivation of p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000)
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999)
Jain, M. et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104 (2002)
Fisher, G. H. et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 15, 3249–3262 (2001)
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002)
Weinstein, I. B. Cancer. Addiction to oncogenes–the Achilles heel of cancer. Science 297, 63–64 (2002)
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001)
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002)
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004)
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004)
Sherr, C. J. Principles of tumor suppression. Cell 116, 235–246 (2004)
Olivier, M. et al. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum. Mutat. 19, 607–614 (2002)
Bykov, V. J. et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nature Med. 8, 282–288 (2002)
Snyder, E. L., Meade, B. R., Saenz, C. C. & Dowdy, S. F. Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS Biol. 2, E36 (2004)
Branda, C. S. & Dymecki, S. M. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004)
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992)
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994)
Indra, A. K. et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ERT and Cre-ERT2 recombinases. Nucleic Acids Res. 27, 4324–4327 (1999)
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999)
Kemp, C. J., Wheldon, T. & Balmain, A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nature Genet. 8, 66–69 (1994)
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005)
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003)
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature advance online publication, doi:10.1038/nature05529 (24 January 2007)
Zhang, L. et al. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 60, 3655–3661 (2000)
Teodoro, J. G., Parker, A. E., Zhu, X. & Green, M. R. p53-mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 313, 968–971 (2006)
Dickins, R. A. et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nature Genet. 37, 1289–1295 (2005)
Christophorou, M. A. et al. Temporal dissection of p53 function in vitro and in vivo. Nature Genet. 37, 718–726 (2005)
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006)
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004)
Haupt, S. & Haupt, Y. Manipulation of the tumor suppressor p53 for potentiating cancer therapy. Semin. Cancer Biol. 14, 244–252 (2004)
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004)
Acknowledgements
We thank N. Willis for helping to generate the LSL mice, H. Zheng for imaging mice, G. Wojtkiewicz for generating the movies with three-dimensional reconstruction, D. Crowley for help with histology, R. Bronson for reviewing the pathology, and M. Hemann for suggestions. A.V. is grateful to D. Ventura and G. Terranova for continuous support and encouragement. This work was supported by the Howard Hughes Medical Institute (T.J.), NCI (T.J., R.W., D.G.K.), and partially by a Cancer Center Support grant from the NCI (M.I.T.), the American Italian Cancer Research Foundation (A.V.), and the Leaf fund (D.G.K.). T.J. is the David H. Koch Professor of Biology and a Daniel K. Ludwig Scholar. D.A.T. is a Rita Allen Foundation Scholar.
Author Contributions A.V., D.G.K. and T.J. designed the experiments and wrote the paper. D.T. generated the p53-LSL mice and M.E.M. generated and characterized the Cre-ERT2 mice, determined the optimal Tamoxifen dosage, assisted with histopathological analysis and commented on the manuscript. A.V., D.G.K. and L.L. derived and characterized the tumour cell lines. A.V. performed the immunostainings, the TUNEL assays the SA-β-Gal stainings and the western blottings. A.V., D.K. and L.L. performed the tamoxifen intraperitoneal injections. E.E.R. derived the MEFs. L.L. and J.N. maintained the mouse colony and genotyped the animals. J.G. and D.G.K. evaluated the magnetic resonance images, J.G. supervised the magnetic resonance imaging, generated the three-dimensional reconstructions and determined tumour volumes. R.W. optimized in vivo imaging protocols, reviewed imaging data, discussed the results, and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary file 1
This file contains Supplementary Methods, Supplementary Figures 1, 3, 5, 6 and 7 with legends. The Supplementary Figures 2 and 4 are represented as movie files. (PDF 3041 kb)
Supplementary file 2
This file contains movies of 3D reconstructions indicated in the main text as Supplementary Figures 2. (AVI 6872 kb)
Supplementary file 3
This file contains movies of 3D reconstructions indicated in the main text as Supplementary Figures 2. (AVI 7219 kb)
Supplementary file 4
This file contains movies of MRIs indicated in the main text as Supplementary Figures. (MOV 169 kb)
Supplementary file 5
This file contains movies of MRIs indicated in the main text as Supplementary Figures 2. (MOV 185 kb)
Supplementary file 6
This file contains movies of MRIs indicated in the main text as Supplementary Figures 2. (MOV 159 kb)
Supplementary file 7
This file contains movies of MRIs indicated in the main text as Supplementary Figures 2. (MOV 163 kb)
Supplementary file 8
This file contains movies of 3D reconstructions indicated in the main text as Supplementary Figures 4. (AVI 6481 kb)
Supplementary file 9
This file contains movies of 3D reconstructions indicated in the main text as Supplementary Figures 4. (AVI 6200 kb)
Supplementary file 10
This file contains movies of MRIs indicated in the main text as Supplementary Figures 4. (MOV 243 kb)
Supplementary file 11
This file contains movies of MRIs indicated in the main text as Supplementary Figures 4. (MOV 341 kb)
Rights and permissions
About this article
Cite this article
Ventura, A., Kirsch, D., McLaughlin, M. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007). https://doi.org/10.1038/nature05541
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05541