Abstract
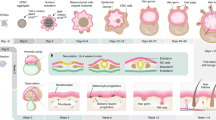
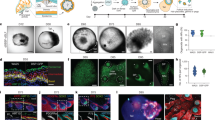
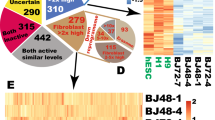
The utility of induced pluripotent stem (iPS) cells for investigating the molecular logic of pluripotency and for eventual clinical application is limited by the low efficiency of current methods for reprogramming. Here we show that reprogramming of juvenile human primary keratinocytes by retroviral transduction with OCT4, SOX2, KLF4 and c-MYC is at least 100-fold more efficient and twofold faster compared with reprogramming of human fibroblasts. Keratinocyte-derived iPS (KiPS) cells appear indistinguishable from human embryonic stem cells in colony morphology, growth properties, expression of pluripotency-associated transcription factors and surface markers, global gene expression profiles and differentiation potential in vitro and in vivo. To underscore the efficiency and practicability of this technology, we generated KiPS cells from single adult human hairs. Our findings provide an experimental model for investigating the bases of cellular reprogramming and highlight potential advantages of using keratinocytes to generate patient-specific iPS cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Lowry, W.E. et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA 105, 2883–2888 (2008).
Park, I.H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Aoi, T. et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321, 699–702 (2008).
Wernig, M. et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 26, 916–924 (2008).
Fuchs, E. Scratching the surface of skin development. Nature 445, 834–842 (2007).
Unsworth, H.C., Aasen, T., McElwaine, S. & Kelsell, D.P. Tissue-specific effects of wild-type and mutant connexin 31: a role in neurite outgrowth. Hum. Mol. Genet. 16, 165–172 (2007).
Hawley, R.G., Lieu, F.H., Fong, A.Z. & Hawley, T.S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1, 136–138 (1994).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 (2008).
O'Connor, M.D. et al. Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells 26, 1109–1116 (2008).
Draper, J.S. et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 22, 53–54 (2004).
Mitalipova, M.M. et al. Preserving the genetic integrity of human embryonic stem cells. Nat. Biotechnol. 23, 19–20 (2005).
Raya, A. et al. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harb. Symp. Quant. Biol. (in press).
Brambrink, T. et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159 (2008).
Assou, S. et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells 25, 961–973 (2007).
Limat, A. & Noser, F.K. Serial cultivation of single keratinocytes from the outer root sheath of human scalp hair follicles. J. Invest. Dermatol. 87, 485–488 (1986).
Kurata, S., Itami, S., Terashi, H. & Takayasu, S. Successful transplantation of cultured human outer root sheath cells as epithelium. Ann. Plast. Surg. 33, 290–294 (1994).
Sridharan, R. & Plath, K. Illuminating the black box of reprogramming. Cell Stem Cell 2, 295–297 (2008).
Segre, J.A., Bauer, C. & Fuchs, E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 22, 356–360 (1999).
Gandarillas, A. & Watt, F.M. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 11, 2869–2882 (1997).
Kim, J.B. et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454, 646–650 (2008).
Amoh, Y., Li, L., Katsuoka, K., Penman, S. & Hoffman, R.M. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc. Natl. Acad. Sci. USA 102, 5530–5534 (2005).
Yu, H. et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am. J. Pathol. 168, 1879–1888 (2006).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
de Hoon, M.J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Freberg, C.T., Dahl, J.A., Timoskainen, S. & Collas, P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol. Biol. Cell 18, 1543–1553 (2007).
Virtaneva, K. et al. Expression profiling reveals fundamental biological differences in acute myeloid leukemia with isolated trisomy 8 and normal cytogenetics. Proc. Natl. Acad. Sci. USA 98, 1124–1129 (2001).
Wu, Z., Irizarry, R.A., Gentleman, R., Martinez-Murillo, F. & Spencer, F. A model-based background adjustment for oligonucleotide expression arrays. J. Am. Stat. Assoc. 99, 909–917 (2004).
Acknowledgements
We are grateful to Ignacio Pizá Rodriguez for help and advice with KiPS cell characterization; José Miguel Andrés Vaquero for assistance with FACS analysis; Meritxell Carrió for expert assistance with cell culture techniques; Esther Melo, Lola Mulero Pérez and Mercé Gaudes Martí for bioimaging assistance; Yvonne Richaud and Teresa Lopez Rovira for excellent technical assistance and Luciano Di Croce, Centre for Genomic Regulation, Barcelona, for the gift of c-MYC T58A plasmid. F.G. was partially supported by a fellowship from the Swiss National Science Foundation. M.J.B. and G.T. were partially supported by the Ramón y Cajal program. This work was partially supported by grants from Ministerio de Educación y Ciencia grant BFU2006-12251, European Commission 'Marie-Curie Reintegration Grant' MIRG-CT-2007-046523 the Fondo de Investigaciones Sanitarias (RETIC-RD06/0010/0016, PI061897), Marató de TV3 (063430), the G. Harold and Leila Y. Mathers Charitable Foundation and Fundación Cellex.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Data (PDF 24671 kb)
Supplementary Movie 1
Real-time movie of KiPS4F4 cells differentiated into beating cardiomyocytes. (MOV 3384 kb)
Supplementary Movie 2
Real-time movie of hair-derived iPS cells differentiated into beating cardiomyocytes (Hair sample 1). (MOV 2843 kb)
Supplementary Movie 3
Real-time movie of hair-derived iPS cells differentiated into beating cardiomyocytes (Hair sample 2). (MOV 2678 kb)
Rights and permissions
About this article
Cite this article
Aasen, T., Raya, A., Barrero, M. et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26, 1276–1284 (2008). https://doi.org/10.1038/nbt.1503
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1503