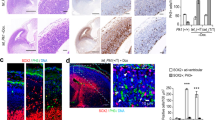
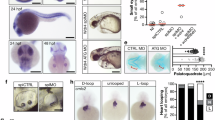
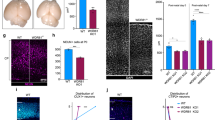
Abstract
Centrioles are essential for ciliogenesis. However, mutations in centriole biogenesis genes have been reported in primary microcephaly and Seckel syndrome, disorders without the hallmark clinical features of ciliopathies. Here we identify mutations in the genes encoding PLK4 kinase, a master regulator of centriole duplication, and its substrate TUBGCP6 in individuals with microcephalic primordial dwarfism and additional congenital anomalies, including retinopathy, thereby extending the human phenotypic spectrum associated with centriole dysfunction. Furthermore, we establish that different levels of impaired PLK4 activity result in growth and cilia phenotypes, providing a mechanism by which microcephaly disorders can occur with or without ciliopathic features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nigg, E.A. & Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 (2009).
Bettencourt-Dias, M., Hildebrandt, F., Pellman, D., Woods, G. & Godinho, S.A. Centrosomes and cilia in human disease. Trends Genet. 27, 307–315 (2011).
Sir, J.H. et al. Loss of centrioles causes chromosomal instability in vertebrate somatic cells. J. Cell Biol. 203, 747–756 (2013).
Ganem, N.J., Godinho, S.A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Gönczy, P. Towards a molecular architecture of centriole assembly. Nat. Rev. Mol. Cell Biol. 13, 425–435 (2012).
Guernsey, D.L. et al. Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am. J. Hum. Genet. 87, 40–51 (2010).
Kalay, E. et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat. Genet. 43, 23–26 (2011).
Bond, J. et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat. Genet. 37, 353–355 (2005).
Kumar, A., Girimaji, S.C., Duvvari, M.R. & Blanton, S.H. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am. J. Hum. Genet. 84, 286–290 (2009).
Sir, J.H. et al. A primary microcephaly protein complex forms a ring around parental centrioles. Nat. Genet. 43, 1147–1153 (2011).
Hussain, M.S. et al. A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. Am. J. Hum. Genet. 90, 871–878 (2012).
Bettencourt-Dias, M. et al. SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 15, 2199–2207 (2005).
Habedanck, R., Stierhof, Y.D., Wilkinson, C.J. & Nigg, E.A. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7, 1140–1146 (2005).
Verloes, A., Drunat, S., Gressens, P. & Passemard, S. in GeneReviews (eds. Pagon, R.A. et al.) 1–2 (University of Washington, Seattle, 1993).
Bahtz, R. et al. GCP6 is a substrate of Plk4 and required for centriole duplication. J. Cell Sci. 125, 486–496 (2012).
Puffenberger, E.G. et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE 7, e28936 (2012).
Hudson, J.W. et al. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr. Biol. 11, 441–446 (2001).
Cizmecioglu, O. et al. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J. Cell Biol. 191, 731–739 (2010).
Kleylein-Sohn, J. et al. Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 (2007).
Hornick, J.E. et al. Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr. Biol. 21, 598–605 (2011).
Bicknell, L.S. et al. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat. Genet. 43, 350–355 (2011).
Pfaff, K.L. et al. The zebrafish cassiopeia mutant reveals that SIL is required for mitotic spindle organization. Mol. Cell. Biol. 27, 5887–5897 (2007).
Coelho, P.A. et al. Spindle formation in the mouse embryo requires Plk4 in the absence of centrioles. Dev. Cell 27, 586–597 (2013).
Izraeli, S. et al. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature 399, 691–694 (1999).
Bazzi, H. & Anderson, K.V. Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo. Proc. Natl. Acad. Sci. USA 111, E1491–E1500 (2014).
McIntyre, R.E. et al. Disruption of mouse Cenpj, a regulator of centriole biogenesis, phenocopies Seckel syndrome. PLoS Genet. 8, e1003022 (2012).
Larison, K.D. & Bremiller, R. Early onset of phenotype and cell patterning in the embryonic zebrafish retina. Development 109, 567–576 (1990).
Wheway, G., Parry, D.A. & Johnson, C.A. The role of primary cilia in the development and disease of the retina. Organogenesis 10, 69–85 (2014).
Blachon, S. et al. Drosophila asterless and vertebrate Cep152 are orthologs essential for centriole duplication. Genetics 180, 2081–2094 (2008).
Slevin, L.K. et al. The structure of the plk4 cryptic polo box reveals two tandem polo boxes required for centriole duplication. Structure 20, 1905–1917 (2012).
Novorol, C. et al. Microcephaly models in the developing zebrafish retinal neuroepithelium point to an underlying defect in metaphase progression. Open Biol. 3, 130065 (2013).
Sillibourne, J.E. & Bornens, M. Polo-like kinase 4: the odd one out of the family. Cell Div. 5, 25 (2010).
Ko, M.A. et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat. Genet. 37, 883–888 (2005).
Holland, A.J. et al. Polo-like kinase 4 controls centriole duplication but does not directly regulate cytokinesis. Mol. Biol. Cell 23, 1838–1845 (2012).
Holland, A.J., Lan, W., Niessen, S., Hoover, H. & Cleveland, D.W. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J. Cell Biol. 188, 191–198 (2010).
Lancaster, M.A. & Knoblich, J.A. Spindle orientation in mammalian cerebral cortical development. Curr. Opin. Neurobiol. 22, 737–746 (2012).
Chen, J.F. et al. Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat. Commun. 5, 3885 (2014).
Thompson, S.L. & Compton, D.A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008).
Marthiens, V. et al. Centrosome amplification causes microcephaly. Nat. Cell Biol. 15, 731–740 (2013).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004).
Sorokin, S.P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207–230 (1968).
Shinohara, K. et al. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat. Commun. 3, 622 (2012).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McLaren, W. et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 26, 2069–2070 (2010).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Yeo, G. & Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 11, 377–394 (2004).
Gudbjartsson, D.F., Jonasson, K., Frigge, M.L. & Kong, A. Allegro, a new computer program for multipoint linkage analysis. Nat. Genet. 25, 12–13 (2000).
Hussain, M.S. et al. CDK6 associates with the centrosome during mitosis and is mutated in a large Pakistani family with primary microcephaly. Hum. Mol. Genet. 22, 5199–5214 (2013).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Wolff, A. et al. Distribution of glutamylated α and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 59, 425–432 (1992).
Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B. & Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Acknowledgements
We thank the families and clinicians for their involvement and participation; P. Mills, T. Hurd, M. Bettencourt-Dias and M. Reijns for commenting on the manuscript; N. Hastie, D. Fitzpatrick and J. Livingston for helpful discussions; C. Janke (Institut Curie) for his kind gift of the GT335 antibody; E. Freyer for assistance with FACS analysis; P. Gautier for bioinformatics; P. Carroll and A. Vickers for technical assistance; the IGMM core sequencing service; the IGMM imaging facility for assistance with microscopy; E. Patton and the IGMM fish facility for advice and zebrafish technical assistance; E. Liston and the DNA Resource Centre at SickKids for sample processing; A. Pearce and E. Maher (Cytogenetics Laboratory, South East Scotland Genetics Service) for technical advice; G. Hahn (University Hospital Carl Gustav Carus) for her second opinion on the MRI data; and N. Dalibor and E. Kirst (CCG) for their expert technical assistance. This work was supported by funding from the MRC, the Lister Institute for Preventative Medicine and the European Research Council (ERC, 281847) (A.P.J.), Medical Research Scotland (L.S.B.), the National Institute for Health Research Moorfields Eye Hospital Biomedical Research Centre (A.T.M.), Köln Fortune (M.S.H.) and CMMC (P.N. and A.A.N.).
Author information
Authors and Affiliations
Contributions
P.N., H.T., J.A., M.S.H., A.B., K.M., M.E. Hurles, J.E.M. and L.S.B. performed exome sequencing and analysis. L.S.B., C.-A.M., J.E.M., M.R.T., I.A., M.S.H. and G.N. performed sequencing, genotyping, linkage analysis and other molecular genetics experiments. C.-A.M., A.L., C.K., M.E. Harley, I.A., M.S.H., R.M., A.A.N. and I.H. designed and performed the cell biology experiments. A. Klingseisen designed and performed the zebrafish experiments with help from A.L., C.-A.M., J.D. and P.H. W.H. performed structural analysis. D.H., F.K., Z.A., S.T., V.C.-D., H.D., L.D., A. Kariminejad, R.M.-L., A.T.M., A.S., C.S., R.W. and S.M.B. ascertained subjects, obtained samples and/or assisted with phenotypic analysis and clinical studies. C.-A.M. and A.P.J. wrote the manuscript with help from P.N., A. Klingseisen and L.S.B. The study was planned and supervised by P.N. and A.P.J.
Corresponding authors
Ethics declarations
Competing interests
P.N. is a founder, CEO and shareholder of ATLAS Biolabs. ATLAS Biolabs is a service provider for genomic analyses.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17, Supplementary Tables 1–3 and Supplementary Note. (PDF 3515 kb)
Rights and permissions
About this article
Cite this article
Martin, CA., Ahmad, I., Klingseisen, A. et al. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy. Nat Genet 46, 1283–1292 (2014). https://doi.org/10.1038/ng.3122
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3122
This article is cited by
-
The interleukin-11 receptor variant p.W307R results in craniosynostosis in humans
Scientific Reports (2023)
-
Identification of deleterious variants in patients with male infertility due to idiopathic non-obstructive azoospermia
Reproductive Biology and Endocrinology (2022)
-
Genetic interaction between PLK1 and downstream MCPH proteins in the control of centrosome asymmetry and cell fate during neural progenitor division
Cell Death & Differentiation (2022)
-
Sub-centrosomal mapping identifies augmin-γTuRC as part of a centriole-stabilizing scaffold
Nature Communications (2021)
-
Overexpression of the PLK4 Gene as a Novel Strategy for the Treatment of Autosomal Recessive Microcephaly by Improving Centrosomal Dysfunction
Journal of Molecular Neuroscience (2021)