Abstract
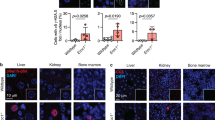
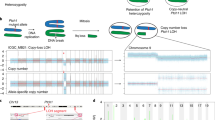
The polo-like kinase Plk4 (also called Sak) is required for late mitotic progression, cell survival and postgastrulation embryonic development1. Here we identified a phenotype resulting from Plk4 haploinsufficiency in Plk4 heterozygous cells and mice. Plk4+/− embryonic fibroblasts had increased centrosomal amplification, multipolar spindle formation and aneuploidy compared with wild-type cells. The incidence of spontaneous liver and lung cancers was ∼15 times high in elderly Plk4+/− mice than in Plk4+/+ littermates. Using the in vivo model of partial hepatectomy to induce synchronous cell cycle entry, we determined that the precise regulation of cyclins D1, E and B1 and of Cdk1 was impaired in Plk4+/− regenerating liver, and p53 activation and p21 and BubR1 expression were suppressed. These defects were associated with progressive cell cycle delays, increased spindle irregularities and accelerated hepatocellular carcinogenesis in Plk4+/− mice. Loss of heterozygosity occurs frequently (∼60%) at polymorphic markers adjacent to the PLK4 locus in human hepatoma. Reduced Plk4 gene dosage increases the probability of mitotic errors and cancer development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hudson, J.W. et al. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr. Biol. 11, 441–446 (2001).
Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 274, 1664–1671 (1996).
Okada, H. & Mak, T.W. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer 4, 592–603 (2004).
Michel, L.S. et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409, 355–359 (2001).
Philipp-Staheli, J., Payne, S.R. & Kemp, C.J. p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 264, 148–168 (2001).
Dai, W. et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 64, 440–445 (2004).
Babu, J.R. et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J. Cell Biol. 160, 341–353 (2003).
Tsai, K.Y. et al. ARF mutation accelerates pituitary tumor development in Rb+/− mice. Proc. Natl. Acad. Sci. USA 99, 16865–16870 (2002).
Jackson, R.J. et al. p21Cip1 nullizygosity increases tumor metastasis in irradiated mice. Cancer Res. 63, 3021–3025 (2003).
Bai, F., Pei, X.H., Godfrey, V.L. & Xiong, Y. Haploinsufficiency of p18(INK4c) sensitizes mice to carcinogen-induced tumorigenesis. Mol. Cell. Biol. 23, 1269–1277 (2003).
Sherr, C.J. Principles of tumor suppression. Cell 116, 235–246 (2004).
Barr, F.A., Sillje, H.H. & Nigg, E.A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5, 429–440 (2004).
Blagden, S.P. & Glover, D.M. Polar expeditions–provisioning the centrosome for mitosis. Nat. Cell Biol. 5, 505–511 (2003).
Lindon, C. & Pines, J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 164, 233–241 (2004).
Fode, C., Binkert, C. & Dennis, J.W. Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol. Cell. Biol. 16, 4665–4672 (1996).
Hammond, C., Jeffers, L., Carr, B.I. & Simon, D. Multiple genetic alterations, 4q28, a new suppressor region, and potential gender differences in human hepatocellular carcinoma. Hepatology 29, 1479–1485 (1999).
Galjart, N. & Perez, F. A plus-end raft to control microtubule dynamics and function. Curr. Opin. Cell Biol. 15, 48–53 (2003).
Black, D., Lyman, S., Heider, T.R. & Behrns, K.E. Molecular and cellular features of hepatic regeneration. J. Surg. Res. 117, 306–315 (2004).
Hixon, M.L. et al. Akt1/PKB upregulation leads to vascular smooth muscle cell hypertrophy and polyploidization. J. Clin. Invest. 106, 1011–1020 (2000).
Tarapore, P. & Fukasawa, K. Loss of p53 and centrosome hyperamplification. Oncogene 21, 6234–6240 (2002).
Seike, M. et al. The promoter region of the human BUBR1 gene and its expression analysis in lung cancer. Lung Cancer 38, 229–234 (2002).
Ferrell, J.E., Jr. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002).
Humbert, P., Russell, S. & Richardson, H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays 25, 542–553 (2003).
Carroll, P.E. et al. Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene 18, 1935–1944 (1999).
Swallow, C. et al. Sak/Plk4 and mitotic fidelity. Oncogene 24, 306–312 (2005).
Hirao, A. et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287, 1824–1827 (2000).
Anzola, M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J. Viral Hepat. 11, 383–393 (2004).
Weinberg, R.A. The cat and mouse games that genes, viruses, and cells play. Cell 88, 573–575 (1997).
Bluteau, O. et al. Specific association between alcohol intake, high grade of differentiation and 4q34-q35 deletions in hepatocellular carcinomas identified by high resolution allelotyping. Oncogene 21, 1225–1232 (2002).
Yeh, S.H. et al. Chromosomal allelic imbalance evolving from liver cirrhosis to hepatocellular carcinoma. Gastroenterology 121, 699–709 (2001).
Acknowledgements
We thank N. DiNicola and A. Sproule for technical assistance and S. Gallinger, I. McGilvray, L. Osborne, S. Scherer, R. Chetty, C McKerlie and T. Pawson for discussions. This work was supported by grants from the National Cancer Institute of Canada (J.W.D.) and the National Colorectal Cancer Campaign (C.J.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Slow growth, mitotic irregularities, and micronuclei in Sak+/− MEFs. (PDF 185 kb)
Supplementary Table 1
PCR primers and probes. (PDF 31 kb)
Rights and permissions
About this article
Cite this article
Ko, M., Rosario, C., Hudson, J. et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet 37, 883–888 (2005). https://doi.org/10.1038/ng1605
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1605