Abstract
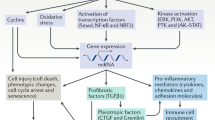
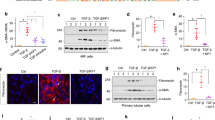
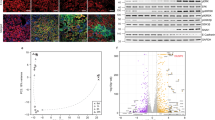
Molecules associated with the transforming growth factor β (TGF-β) superfamily, such as bone morphogenic proteins (BMPs) and TGF-β, are key regulators of inflammation, apoptosis and cellular transitions. Here we show that the BMP receptor activin-like kinase 3 (Alk3) is elevated early in diseased kidneys after injury. We also found that its deletion in the tubular epithelium leads to enhanced TGF-β1–Smad family member 3 (Smad3) signaling, epithelial damage and fibrosis, suggesting a protective role for Alk3-mediated signaling in the kidney. A structure-function analysis of the BMP-Alk3–BMP receptor, type 2 (BMPR2) ligand-receptor complex, along with synthetic organic chemistry, led us to construct a library of small peptide agonists of BMP signaling that function through the Alk3 receptor. One such peptide agonist, THR-123, suppressed inflammation, apoptosis and the epithelial-to-mesenchymal transition program and reversed established fibrosis in five mouse models of acute and chronic renal injury. THR-123 acts specifically through Alk3 signaling, as mice with a _targeted deletion for Alk3 in their tubular epithelium did not respond to therapy with THR-123. Combining THR-123 and the angiotensin-converting enzyme inhibitor captopril had an additive therapeutic benefit in controlling renal fibrosis. Our studies show that BMP signaling agonists constitute a new line of therapeutic agents with potential utility in the clinic to induce regeneration, repair and reverse established fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zeisberg, M. & Kalluri, R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J. Mol. Med. 82, 175–181 (2004).
Zeisberg, M. et al. BMP-7 counteracts TGF-β1–induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 (2003).
Zeisberg, E.M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Miyazono, K., Maeda, S. & Imamura, T. BMP receptor signaling: transcriptional _targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 16, 251–263 (2005).
Simic, P. & Vukicevic, S. Bone morphogenetic proteins: from developmental signals to tissue regeneration. Conference on bone morphogenetic proteins. EMBO Rep. 8, 327–331 (2007).
Simic, P. & Vukicevic, S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 16, 299–308 (2005).
Ishidou, Y. et al. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J. Bone Miner. Res. 10, 1651–1659 (1995).
Bosukonda, D., Shih, M.S., Sampath, K.T. & Vukicevic, S. Characterization of receptors for osteogenic protein-1/bone morphogenetic protein-7 (OP-1/BMP-7) in rat kidneys. Kidney Int. 58, 1902–1911 (2000).
Schainuck, L.I., Striker, G.E., Cutler, R.E. & Benditt, E.P. Structural-functional correlations in renal disease. II. The correlations. Hum. Pathol. 1, 631–641 (1970).
Striker, G.E., Schainuck, L.I., Cutler, R.E. & Benditt, E.P. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum. Pathol. 1, 615–630 (1970).
Risdon, R.A., Sloper, J.C. & de Wardener, H.E. Relationship between renal function and histological changes found in renal biopsy specimens from patients with persistent glomerular nephritis. Lancet 2, 363–366 (1968).
Nath, K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 20, 1–17 (1992).
Mackensen-Haen, S., Bader, R., Grund, K.E. & Bohle, A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin. Nephrol. 15, 167–171 (1981).
Sugimoto, H., Grahovac, G., Zeisberg, M. & Kalluri, R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56, 1825–1833 (2007).
Zeisberg, M., Shah, A.A. & Kalluri, R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J. Biol. Chem. 280, 8094–8100 (2005).
Zeisberg, M. et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am. J. Physiol. Renal Physiol. 285, F1060–F1067 (2003).
Tuglular, S. et al. Cyclosporine-A induced nephrotoxicity is associated with decreased renal bone morphogenetic protein-7 expression in rats. Transplant. Proc. 36, 131–133 (2004).
Wang, S.N., Lapage, J. & Hirschberg, R. Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J. Am. Soc. Nephrol. 12, 2392–2399 (2001).
Vukicevic, S. et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Invest. 102, 202–214 (1998).
Simon, M. et al. Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am. J. Physiol. 276, F382–F389 (1999).
Srinivas, S. et al. Cre reporter strains produced by _targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Mishina, Y., Hanks, M.C., Miura, S., Tallquist, M.D. & Behringer, R.R. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32, 69–72 (2002).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Rodríguez-Iturbe, B. & Garcia Garcia, G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin. Pract. 116, c81–c88 (2010).
Schlunegger, M.P. & Grutter, M.G. An unusual feature revealed by the crystal structure at 2.2 Å resolution of human transforming growth factor-β 2. Nature 358, 430–434 (1992).
Daopin, S., Piez, K.A., Ogawa, Y. & Davies, D.R. Crystal structure of transforming growth factor-β 2: an unusual fold for the superfamily. Science 257, 369–373 (1992).
Griffith, D.L., Keck, P.C., Sampath, T.K., Rueger, D.C. & Carlson, W.D. Three-dimensional structure of recombinant human osteogenic protein 1: structural paradigm for the transforming growth factor β superfamily. Proc. Natl. Acad. Sci. USA 93, 878–883 (1996).
Keck, P.C. Computer method and apparatus for classifying objects. United States Patent Office patent WO/2002/037313 (2002).
Gould, S.E., Day, M., Jones, S.S. & Dorai, H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 61, 51–60 (2002).
Lloyd, C.M. et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 185, 1371–1380 (1997).
Kashtan, C.E. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 78, 338–360 (1999).
Parving, H.H., Hommel, E., Damkjaer Nielsen, M. & Giese, J. Effect of captopril on blood pressure and kidney function in normotensive insulin dependent diabetics with nephropathy. Br. Med. J. 299, 533–536 (1989).
Lewis, E.J., Hunsicker, L.G., Bain, R.P. & Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Border, W.A. & Noble, N.A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292 (1994).
Hojo, H., Ohba, S., Yano, F. & Chung, U.I. Coordination of chondrogenesis and osteogenesis by hypertrophic chondrocytes in endochondral bone development. J. Bone Miner. Metab. 28, 489–502 (2010).
Archdeacon, P. & Detwiler, R.K. Bone morphogenetic protein 7 (BMP7): a critical role in kidney development and a putative modulator of kidney injury. Adv. Chronic Kidney Dis. 15, 314–320 (2008).
Haaijman, A. et al. Correlation between ALK-6 (BMPR-IB) distribution and responsiveness to osteogenic protein-1 (BMP-7) in embryonic mouse bone rudiments. Growth Factors 17, 177–192 (2000).
Ide, H. et al. Growth regulation of human prostate cancer cells by bone morphogenetic protein-2. Cancer Res. 57, 5022–5027 (1997).
Wetzel, P. et al. Bone morphogenetic protein-7 expression and activity in the human adult normal kidney is predominantly localized to the distal nephron. Kidney Int. 70, 717–723 (2006).
Stenbicker, A.U. et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood 118, 4224–4230 (2011).
Acknowledgements
This study was supported by US National Institutes of Health grants DK55001, DK061688, DK081576, DK074558, CA155370 CA163191, CA125550, CA151925 and the Else Kröner–Memorial–Stipend P58/05//EKMS 05/59 (M.Z.). V.S.L. is funded from the Research Training Grant in Gastroenterology (2T32DK007760–11). H.S. is funded by the Research Training Grant in Cardiovascular Biology (5T32HL007374–30). Mice with the Alk3flox/flox allele (Alk3f/f) were kindly provided by Y. Mishina, US National Institutes of Health under a material transfer agreement. γGT-Cre mice, NP-1 cells and the polyclonal antibody to FSP1 were provided by E. Neilson, Vanderbilt University Medical Center. We thank S. McGoohan for his technical assistance.
Author information
Authors and Affiliations
Contributions
R.K. conceptually designed the strategy for this study, participated in discussions, provided intellectual input, supervised the studies and wrote the manuscript. H.S. performed experiments and analyzed the data. M.Z. participated in the discussions and performed experiments. W.B., P.K., D.B., W.C., M.R., P.B., G.T., H.O., D.T., B.T. and V.S.L. performed experiments, analyzed the data and edited the manuscript and generated the figures. K.K. and V.S.L. supervised in vitro experiments, helped in the writing of the manuscript and generated the figures.
Corresponding author
Ethics declarations
Competing interests
P.K., D.B., R.K., W.C. and P.B. hold equity in Thrasos, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1620 kb)
Rights and permissions
About this article
Cite this article
Sugimoto, H., LeBleu, V., Bosukonda, D. et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 18, 396–404 (2012). https://doi.org/10.1038/nm.2629
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2629