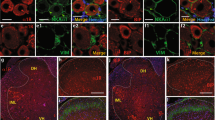
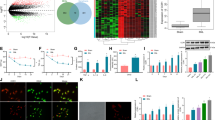
Abstract
Neuropathic pain is a major, intractable clinical problem and its pathophysiology is not well understood. Although recent gene expression profiling studies have enabled the identification of novel _targets for pain therapy1,2,3,4, classical study designs provide unclear results owing to the differential expression of hundreds of genes across sham and nerve-injured groups, which can be difficult to validate, particularly with respect to the specificity of pain modulation5. To circumvent this, we used two outbred lines of rats6, which are genetically similar except for being genetically segregated as a result of selective breeding for differences in neuropathic pain hypersensitivity7. SerpinA3N, a serine protease inhibitor, was upregulated in the dorsal root ganglia (DRG) after nerve injury, which was further validated for its mouse homolog. Mice lacking SerpinA3N developed more neuropathic mechanical allodynia than wild-type (WT) mice, and exogenous delivery of SerpinA3N attenuated mechanical allodynia in WT mice. T lymphocytes infiltrate the DRG after nerve injury and release leukocyte elastase (LE), which was inhibited by SerpinA3N derived from DRG neurons. Genetic loss of LE or exogenous application of a LE inhibitor (Sivelastat) in WT mice attenuated neuropathic mechanical allodynia. Overall, we reveal a novel and clinically relevant role for a member of the serpin superfamily and a leukocyte elastase and crosstalk between neurons and T cells in the modulation of neuropathic pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Costigan, M. et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain 133, 2519–2527 (2010).
Nissenbaum, J. et al. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res. 20, 1180–1190 (2010).
Sorge, R.E. et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 18, 595–599 (2012).
Tegeder, I. et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat. Med. 12, 1269–1277 (2006).
Persson, A.K. et al. Correlational analysis for identifying genes whose regulation contributes to chronic neuropathic pain. Mol. Pain 5, 7 (2009).
Devor, M. & Raber, P. Heritability of symptoms in an experimental model of neuropathic pain. Pain 42, 51–67 (1990).
Ziv-Sefer, S., Raber, P., Barbash, S. & Devor, M. Unity vs. diversity of neuropathic pain mechanisms: allodynia and hyperalgesia in rats selected for heritable predisposition to spontaneous pain. Pain 146, 148–157 (2009).
Seijffers, R., Mills, C.D. & Woolf, C.J. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J. Neurosci. 27, 7911–7920 (2007).
Horvath, A.J. et al. The murine orthologue of human antichymotrypsin: a structural paradigm for clade A3 serpins. J. Biol. Chem. 280, 43168–43178 (2005).
Sipione, S. et al. Identification of a novel human granzyme B inhibitor secreted by cultured sertoli cells. J. Immunol. 177, 5051–5058 (2006).
Gettins, P.G. Serpin structure, mechanism, and function. Chem. Rev. 102, 4751–4804 (2002).
Lee, W.L. & Downey, G.P. Leukocyte elastase: physiological functions and role in acute lung injury. Am. J. Respir. Crit. Care Med. 164, 896–904 (2001).
Pham, C.T. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 6, 541–550 (2006).
García, P.S., Gulati, A. & Levy, J.H. The role of thrombin and protease-activated receptors in pain mechanisms. Thromb. Haemost. 103, 1145–1151 (2010).
Kawasaki, Y. et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 14, 331–336 (2008).
Ferry, G. et al. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett. 402, 111–115 (1997).
Jackson, P.L. et al. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol. Med. 16, 159–166 (2010).
Kawabata, K. et al. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem. Biophys. Res. Commun. 177, 814–820 (1991).
McMahon, S.B. & Malcangio, M. Current challenges in glia-pain biology. Neuron 64, 46–54 (2009).
Scholz, J. & Woolf, C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10, 1361–1368 (2007).
Hu, P., Bembrick, A.L., Keay, K.A. & McLachlan, E.M. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav. Immun. 21, 599–616 (2007).
Kim, C.F. & Moalem-Taylor, G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 1405, 95–108 (2011).
Gehrig, S., Mall, M.A. & Schultz, C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew. Chem. Int. Edn Engl. 51, 6258–6261 (2012).
Costigan, M. et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 29, 14415–14422 (2009).
Irving, J.A., Pike, R.N., Lesk, A.M. & Whisstock, J.C. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 10, 1845–1864 (2000).
Irving, J.A. et al. Serpins in prokaryotes. Mol. Biol. Evol. 19, 1881–1890 (2002).
Alam, S.R., Newby, D.E. & Henriksen, P.A. Role of the endogenous elastase inhibitor, elafin, in cardiovascular injury: from epithelium to endothelium. Biochem. Pharmacol. 83, 695–704 (2012).
Reardon, C. et al. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity 35, 223–235 (2011).
Belaaouaj, A. et al. Mice lacking neutrophil elastase reveal impaired host defense against Gram-negative bacterial sepsis. Nat. Med. 4, 615–618 (1998).
Griffin, R.S. et al. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J. Neurosci. 27, 8699–8708 (2007).
Bolstad, B.M., Irizarry, R.A., Astrand, M. & Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003).
Irizarry, R.A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Gentleman, R.C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Díaz, E. et al. Molecular analysis of gene expression in the developing pontocerebellar projection system. Neuron 36, 417–434 (2002).
Decosterd, I. & Woolf, C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000).
Luo, C. et al. Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. PLoS Biol. 10, e1001283 (2012).
Costigan, M. et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 3, 16 (2002).
Blackshaw, S. & Snyder, S.H. Parapinopsin, a novel catfish opsin localized to the parapineal organ, defines a new gene family. J. Neurosci. 17, 8083–8092 (1997).
Montmayeur, J.P., Liberles, S.D., Matsunami, H. & Buck, L.B. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 4, 492–498 (2001).
Schaeper, U. et al. Distinct requirements for Gab1 in Met and EGF receptor signaling in vivo. Proc. Natl. Acad. Sci. USA 104, 15376–15381 (2007).
Lu, J. et al. Pain in experimental autoimmune encephalitis: a comparative study between different mouse models. J. Neuroinflammation 9, 233 (2012).
Basso, A.S. et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 118, 1532–1543 (2008).
Kim, C.F. & Moalem-Taylor, G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 1405, 95–108 (2011).
Jiang, P. et al. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat. Commun. 4, 2196 (2013).
Gangadharan, V. et al. A novel biological role for the phospholipid lysophosphatidylinositol in nociceptive sensitization via activation of diverse G protein signalling pathways in sensory nerves in vivo. Pain 154, 2801–2812 (2013).
Xu, Q. et al. In vivo gene knockdown in rat dorsal root ganglia mediated by self-complementary adeno-associated virus serotype 5 following intrathecal delivery. PLoS ONE 7, e32581 (2012).
Wang, Z. et al. Differentiation of neuronal cells from NIH/3T3 fibroblasts under defined conditions. Dev. Growth Differ. 53, 357–365 (2011).
Hirota, Y. et al. Roles of planar cell polarity signaling in maturation of neuronal precursor cells in the postnatal mouse olfactory bulb. Stem Cells 30, 1726–1733 (2012).
Chiang, H.S. et al. GEF-H1 controls microtubule-dependent sensing of nucleic acids for antiviral host defenses. Nat. Immunol. 15, 63–71 (2014).
Allman, D., Li, J. & Hardy, R.R. Commitment to the B lymphoid lineage occurs before DH-JH recombination. J. Exp. Med. 189, 735–740 (1999).
Ko, S.Y. et al. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 175, 3309–3317 (2005).
Charles, N., Hardwick, D., Daugas, E., Illei, G.G. & Rivera, J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat. Med. 16, 701–707 (2010).
Andoniou, C.E. et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 6, 1011–1019 (2005).
Papatriantafyllou, M. et al. Dickkopf-3, an immune modulator in peripheral CD8 T-cell tolerance. Proc. Natl. Acad. Sci. USA 109, 1631–1636 (2012).
Gehrig, S., Mall, M.A. & Schultz, C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew. Chem. Int. Edn Engl. 51, 6258–6261 (2012).
Acknowledgements
The authors are grateful to R. LeFaucheur for secretarial assistance and to D. Baumgartl-Ahlert for technical assistance. We acknowledge support from the Interdisciplinary Neurobehavioral Core, Heidelberg, for the behavioral experiments performed here. We are grateful to M. Meister for his help in the analysis of FACS data. This work was supported by a European Research Council (ERC) Advanced Investigator Grant (PAINPLASTICITY; project no. 294293) to R.K., by grants from the Deutsche Forschungsgemeinschaft (DFG; MA 2081/4-1) to M.A.M., by grants from the Deutsche Forschungsgemeinschaft (SFB 938) to B.A., by grants from the US National Institutes of Health (5 R01 NS038253 and 2R37NS039518 to C.W., and R01 NS074430 to M.C.), and by a grant from the Israel Science Foundation to M.D. M.A.M. and R.K. are members of the Molecular Medicine Partnership Unit, Heidelberg. R.K. is a principal investigator in the Excellence Cluster 'CellNetworks' of Heidelberg University. M.A.M. is a member of the German Center for Lung Research. L.V. was partially supported by a PhD fellowship from CellNetworks and by the Hartmut-Hoffmann Berling International Graduate School for Cellular and Molecular Biology. M.S. was partially supported by a post-doctoral fellowship from CellNetworks. A.L. was partially supported by the RO1DE022912 grant from the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
L.V., D.E.S., A.L., K.K.B., M.S., D.H., S.P., P.R., R.S.G., C.N., S.G. and M.C. performed experiments and analyzed data; R.K., M.C., C.J.W., S.D.L., M.D., B.A., M.A.M. designed and supervised experiments; R.K., L.V. and M.C. primarily wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have a patent application pending based on the data herein on the use of leukocyte elastase inhibitors against pain of neuropathic origin (patent application number: EP14200012.4 at the European Patent Office).
Supplementary information
Supplementary Text and Figures
Supplementary Notes 1 and 2, Supplementary Table legends 1 and 2 and Supplementary Figures 1–12 (PDF 5934 kb)
Rights and permissions
About this article
Cite this article
Vicuña, L., Strochlic, D., Latremoliere, A. et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell–derived leukocyte elastase. Nat Med 21, 518–523 (2015). https://doi.org/10.1038/nm.3852
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3852