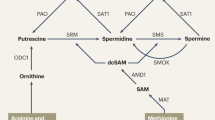
Abstract
Aging is associated with an increased risk of cardiovascular disease and death. Here we show that oral supplementation of the natural polyamine spermidine extends the lifespan of mice and exerts cardioprotective effects, reducing cardiac hypertrophy and preserving diastolic function in old mice. Spermidine feeding enhanced cardiac autophagy, mitophagy and mitochondrial respiration, and it also improved the mechano-elastical properties of cardiomyocytes in vivo, coinciding with increased titin phosphorylation and suppressed subclinical inflammation. Spermidine feeding failed to provide cardioprotection in mice that lack the autophagy-related protein Atg5 in cardiomyocytes. In Dahl salt-sensitive rats that were fed a high-salt diet, a model for hypertension-induced congestive heart failure, spermidine feeding reduced systemic blood pressure, increased titin phosphorylation and prevented cardiac hypertrophy and a decline in diastolic function, thus delaying the progression to heart failure. In humans, high levels of dietary spermidine, as assessed from food questionnaires, correlated with reduced blood pressure and a lower incidence of cardiovascular disease. Our results suggest a new and feasible strategy for protection against cardiovascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Zile, M.R. & Brutsaert, D.L. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis and measurements of diastolic function. Circulation 105, 1387–1393 (2002).
Chiao, Y.A. & Rabinovitch, P.S. The aging heart. Cold Spring Harb. Perspect. Med. 5, a025148 (2015).
Redfield, M.M. et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart-failure epidemic. J. Am. Med. Assoc. 289, 194–202 (2003).
Bui, A.L., Horwich, T.B. & Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 8, 30–41 (2011).
Nakai, A. et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 13, 619–624 (2007).
Taneike, M. et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600–606 (2010).
Madeo, F., Zimmermann, A., Maiuri, M.C. & Kroemer, G. Essential role for autophagy in lifespan extension. J. Clin. Invest. 125, 85–93 (2015).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Morselli, E. et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 192, 615–629 (2011).
Gupta, V.K. et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460 (2013).
Büttner, S. et al. Spermidine protects against α-synuclein neurotoxicity. Cell Cycle 13, 3903–3908 (2014).
Wang, I.-F. et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc. Natl. Acad. Sci. USA 109, 15024–15029 (2012).
Weindruch, R., Walford, R.L., Fligiel, S. & Guthrie, D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 116, 641–654 (1986).
Dai, D.-F. et al. Overexpression of catalase _targeted to mitochondria attenuates murine cardiac aging. Circulation 119, 2789–2797 (2009).
Blackwell, B.N., Bucci, T.J., Hart, R.W. & Turturro, A. Longevity, body weight and neoplasia in ad libitum–fed and diet-restricted C57BL6 mice fed NIH-31 open-formula diet. Toxicol. Pathol. 23, 570–582 (1995).
Treuting, P.M. et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 63, 813–822 (2008).
Soda, K., Kano, Y., Chiba, F., Koizumi, K. & Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS One 8, e64357 (2013).
Paulus, W.J. et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 28, 2539–2550 (2007).
Ky, B. et al. Ventricular-arterial coupling, remodeling and prognosis in chronic heart failure. J. Am. Coll. Cardiol. 62, 1165–1172 (2013).
Dai, D.-F. & Rabinovitch, P.S. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc. Med. 19, 213–220 (2009).
Heinzel, F.R., Hohendanner, F., Jin, G., Sedej, S. & Edelmann, F. Myocardial hypertrophy and its role in heart failure with preserved ejection fraction. J. Appl. Physiol. 119, 1233–1242 (2015).
Linke, W.A. & Hamdani, N. Gigantic business: titin properties and function through thick and thin. Circ. Res. 114, 1052–1068 (2014).
Borbély, A. et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation 111, 774–781 (2005).
López-Otín, C., Blasco, M.A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Salvioli, S. et al. Inflamm-aging, cytokines and aging: state-of-the-art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 12, 3161–3171 (2006).
Duicu, O.M. et al. Ageing-induced decrease in cardiac mitochondrial function in healthy rats. Can. J. Physiol. Pharmacol. 91, 593–600 (2013).
Liu, Y., Samuel, B.S., Breen, P.C. & Ruvkun, G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 508, 406–410 (2014).
Yeganeh, B. et al. _targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol. Ther. 143, 87–110 (2014).
Paulus, W.J. & Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Haspel, J. et al. Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy 7, 629–642 (2011).
Hariharan, N., Zhai, P. & Sadoshima, J. Oxidative stress stimulates autophagic flux during ischemia–reperfusion. Antioxid. Redox Signal. 14, 2179–2190 (2011).
Shirakabe, A. et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure-overload-induced mitochondrial dysfunction and heart failure. Circulation 133, 1249–1263 (2016).
Gottdiener, J.S. et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J. Am. Coll. Cardiol. 35, 1628–1637 (2000).
Doi, R. et al. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J. Hypertens. 18, 111–120 (2000).
Qu, P. et al. Time-course changes in left ventricular geometry and function during the development of hypertension in Dahl salt-sensitive rats. Hypertens. Res. 23, 613–623 (2000).
Palmer, R.M., Ashton, D.S. & Moncada, S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature 333, 664–666 (1988).
Chen, P.Y. & Sanders, P.W. l-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J. Clin. Invest. 88, 1559–1567 (1991).
Tang, W.H.W., Wang, Z., Cho, L., Brennan, D.M. & Hazen, S.L. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J. Am. Coll. Cardiol. 53, 2061–2067 (2009).
Sourij, H. et al. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 218, 220–225 (2011).
Ommen, S.R. et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler–catheterization study. Circulation 102, 1788–1794 (2000).
Kelly, R.P. et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 86, 513–521 (1992).
Leoncini, G. et al. Renal and cardiac abnormalities in primary hypertension. J. Hypertens. 27, 1064–1073 (2009).
Gori, M. et al. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur. Heart J. 35, 3442–3451 (2014).
Klotz, S. et al. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension 47, 901–911 (2006).
Mori, K. & Nakao, K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 71, 967–970 (2007).
Stegemann, C. et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 129, 1821–1831 (2014).
Schindler, C.E., Partap, U., Patchen, B.K. & Swoap, S.J. Chronic rapamycin treatment causes diabetes in male mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R434–R443 (2014).
Miller, R.A. et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477 (2014).
LaRocca, T.J., Gioscia-Ryan, R.A., Hearon, C.M. Jr. & Seals, D.R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 134, 314–320 (2013).
García-Prat, L. et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Wettschureck, N. et al. Absence of pressure-overload-induced myocardial hypertrophy after conditional inactivation of Gαq–Gα11 in cardiomyocytes. Nat. Med. 7, 1236–1240 (2001).
Sedej, S. et al. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc. Res. 87, 50–59 (2010).
Miller, R.A. et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell 6, 565–575 (2007).
Yuan, R. et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8, 277–287 (2009).
Kastenmayer, R.J., Fain, M.A. & Perdue, K.A. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J. Am. Assoc. Lab. Anim. Sci. 45, 8–12 (2006).
Rozman, J. et al. Glucose tolerance tests for systematic screening of glucose homeostasis in mice. Curr. Protoc. Mouse Biol. 5, 65–84 (2015).
Sedej, S. et al. Subclinical abnormalities in sarcoplasmic reticulum Ca2+ release promote eccentric myocardial remodeling and pump failure death in response to pressure overload. J. Am. Coll. Cardiol. 63, 1569–1579 (2014).
Troy, B.L., Pombo, J. & Rackley, C.E. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation 45, 602–611 (1972).
Pacher, P., Nagayama, T., Mukhopadhyay, P., Bátkai, S. & Kass, D.A. Measurement of cardiac function using pressure–volume conductance catheter technique in mice and rats. Nat. Protoc. 3, 1422–1434 (2008).
Abdellatif, M. et al. Spectral transfer function analysis of respiratory hemodynamic fluctuations predicts end-diastolic stiffness in preserved ejection fraction heart failure. Am. J. Physiol. Heart Circ. Physiol. 310, H4–H13 (2016).
Tournoux, F. et al. Validation of noninvasive measurements of cardiac output in mice using echocardiography. J. Am. Soc. Echocardiogr. 24, 465–470 (2011).
Wolf, D. et al. CD4+CD25+ regulatory T cells inhibit experimental anti–glomerular basement membrane glomerulonephritis in mice. J. Am. Soc. Nephrol. 16, 1360–1370 (2005).
Saeed, A.I. et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34, 374–378 (2003).
Sturn, A., Quackenbush, J. & Trajanoski, Z. Genesis: cluster analysis of microarray data. Bioinformatics 18, 207–208 (2002).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J.V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micropurification, enrichment, prefractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007).
Sprenger, A., Küttner, V., Bruckner-Tuderman, L. & Dengjel, J. Global proteome analyses of SILAC-labeled skin cells. Methods Mol. Biol. 961, 179–191 (2013).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Magnes, C. et al. Polyamines in biological samples: rapid and robust quantification by solid-phase extraction online-coupled to liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1331, 44–51 (2014).
Yuan, M., Breitkopf, S.B., Yang, X. & Asara, J.M. A positive/negative ion-switching, _targeted mass-spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7, 872–881 (2012).
Braun, R.J. et al. Accumulation of basic amino acids at mitochondria dictates the cytotoxicity of aberrant ubiquitin. Cell Rep. 10, 1557–1571 (2015).
Buescher, J.M., Moco, S., Sauer, U. & Zamboni, N. Ultrahigh-performance liquid chromatography–tandem mass spectrometry method for fast and robust quantification of anionic and aromatic metabolites. Anal. Chem. 82, 4403–4412 (2010).
Roth, M. Fluorescence reaction for amino acids. Anal. Chem. 43, 880–882 (1971).
Schwarz, E.L., Roberts, W.L. & Pasquali, M. Analysis of plasma amino acids by HPLC with photodiode array and fluorescence detection. Clin. Chim. Acta 354, 83–90 (2005).
Shirakabe, A. et al. Evaluating mitochondrial autophagy in the mouse heart. J. Mol. Cell. Cardiol. 92, 134–139 (2016).
Neagoe, C. et al. Titin isoform switch in ischemic human heart disease. Circulation 106, 1333–1341 (2002).
Hamdani, N. et al. Crucial role for Ca2+–calmodulin-dependent protein kinase II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ. Res. 112, 664–674 (2013).
Mayhew, T.M. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta 29, 1–14 (2008).
Mühlfeld, C., Nyengaard, J.R. & Mayhew, T.M. A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc. Pathol. 19, 65–82 (2010).
Méndez, J. & Keys, A. Density and composition of mammalian muscle. Metabolism 9, 184–188 (1960).
Willett, W.C. et al. Reproducibility and validity of a semiquantitative food-frequency questionnaire. Am. J. Epidemiol. 122, 51–65 (1985).
McKee, P.A., Castelli, W.P., McNamara, P.M. & Kannel, W.B. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 285, 1441–1446 (1971).
Willeit, P. et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J. Am. Coll. Cardiol. 64, 851–860 (2014).
Willett, W. & Stampfer, M.J. Total energy intake: implications for epidemiologic analyses. Am. J. Epidemiol. 124, 17–27 (1986).
Assarsson, E. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity and excellent scalability. PLoS One 9, e95192 (2014).
Wei, J., Carroll, R.J., Harden, K.K. & Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42, 2031–2035 (2012).
Burkhoff, D., Mirsky, I. & Suga, H. Assessment of systolic and diastolic ventricular properties via pressure–volume analysis: a guide for clinical, translational and basic researchers. Am. J. Physiol. Heart Circ. Physiol. 289, H501–H512 (2005).
Acknowledgements
We thank N. Mizushima (University of Tokyo) for providing Atg5fl/fl mice and K. Chien (Harvard University) for providing MLC2a-Cre mice. We are grateful to R. Schreiber for assistance with high-resolution respirometry. F.M. is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B12, P24381, P 27893, I1000 and 'SFB Lipotox', as well as to BMWFW and the Karl-Franzens University for grant 'Unkonventionelle Forschung'. S. Sedej is supported by the Austrian Science Fund FWF through grant P27637-B28 and by a grant from the Austrian Heart Foundation (Österreichischer Herzfonds). T.E. is recipient of an APART fellowship from the Austrian Academy of Sciences. M.A. received funding from the FWF (grant P27637-B28) and was trained within the frame of the Ph.D Program Molecular Medicine of the Medical University of Graz. S.B. is supported by the Austrian Science Fund FWF (grant P27183-B24) and the Swedish Research Council (grant 2015-05468). J.D. is supported by the DFG via grant CRC1140 and by the Swiss National Science Foundation, grant 31003A-166482/1. P.R. is supported by the Austrian Science Fund (FWF) project J3742-B28 and NAWI Graz. W.A.L. is supported by EU (FP7) program MEDIA and the German Research Foundation grant SFB1002, TPA8. G.K. is supported by the LeDucq Foundation, the Cancéropôle Ile-de-France, the Institut National du Cancer (INCa), the European Research Council (ERC), LabEx Immuno-Oncology and the Paris Alliance of Cancer Research Institutes (PACRI). The project was supported by grants from the Helmholtz Portfolio Theme 'Metabolic Dysfunction and Common Disease' (J.B.), the Helmholtz Alliance ('Imaging and Curing Environmental Metabolic Diseases (ICEMED)'; J.B.) and the German Federal Ministry of Education and Research (Infrafrontier grant 01KX1012) (M.H.d.A.). S.J.S. was supported by grants from the Bundesministerium für Bildung und Forschung (Smartage, 01GQ1420A), the Forschungszentrum für neurodegenerative Erkrankungen and the Deutsche Forschungsgemeinschaft (Exc 257). S.K., J.W., R.P., P.W. and M.M. are supported by an excellence initiative (Competence Centers for Excellent Technologies; COMET) of the Austrian Research Promotion Agency FFG: 'Research Center of Excellence in Vascular Ageing–Tyrol, VASCage' (K-Project Nr. 843536) funded by the BMVIT, BMWFW, the Wirtschaftsagentur Wien and the Standortagentur Tirol. This work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas's NHS Foundation Trust and King's College London in partnership with King's College Hospital. M.M. is a Senior Research fellow of the British Heart Foundation. The authors are grateful for the support by staff members of the animal facilities of the Institutes of Biomedical Research (IBF, Medical University of Graz) and Molecular Biosciences (IMB, University of Graz) and acknowledge the Center for Medical Research (ZMF) of the Medical University of Graz for assistance.
Author information
Authors and Affiliations
Contributions
T.E., S. Sedej, G.K. and F.M. designed and supervised the study; T.E., M.A., G.K., S. Sedej and F.M. wrote the manuscript; T.E., M.A., S. Schroeder, U.P., S. Stekovic, T. Pendl, A.H., J. Schipke, A.Z., A.S., M.T., C.R., C.D., A.S.G., V.H., C. Magnes, G.T., S.N., A.M., Z.H., A.K., D.C.-G., S.B., F.P., O.K., E.S., P.R., C.S., A.R., M.H., F.N., D.J., B.R., J.R, T.M., M.M., P.W., M.v.F.-S., R.P. and S. Sedej performed experiments and analyzed and discussed data; K.E., K.M., J.B., H.F., V.G.-D., M.H.d.A., G.H., B.P., L.S., T. Pieber, J.W., S.J.S., W.A.L., C. Mühlfeld, J. Sadoshima, J.D. and S.K. discussed and analyzed data and gave conceptual advice.
Corresponding authors
Ethics declarations
Competing interests
F.M., T.E., D.C.-G., S.J.S. and S. Stekovic have equity interests in TLL, a company founded in 2016 that will develop natural food extracts.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18, Supplementary Tables 1–16, Supplementary Notes (PDF 16921 kb)
Rights and permissions
About this article
Cite this article
Eisenberg, T., Abdellatif, M., Schroeder, S. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22, 1428–1438 (2016). https://doi.org/10.1038/nm.4222
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4222
This article is cited by
-
Polyamines: their significance for maintaining health and contributing to diseases
Cell Communication and Signaling (2023)
-
Un_targeted metabolomic analysis of ischemic injury in human umbilical vein endothelial cells reveals the involvement of arginine metabolism
Nutrition & Metabolism (2023)
-
Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging
Nature Aging (2023)
-
Spermidine promotes fertility in aged female mice
Nature Aging (2023)
-
Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice
Scientific Reports (2023)