Abstract
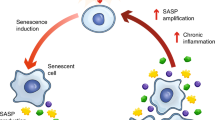
Aging is associated with increased cellular senescence, which is hypothesized to drive the eventual development of multiple comorbidities1. Here we investigate a role for senescent cells in age-related bone loss through multiple approaches. In particular, we used either genetic (i.e., the INK-ATTAC 'suicide' transgene encoding an inducible caspase 8 expressed specifically in senescent cells2,3,4) or pharmacological (i.e., 'senolytic' compounds5,6) means to eliminate senescent cells. We also inhibited the production of the proinflammatory secretome of senescent cells using a JAK inhibitor (JAKi)3,7. In aged (20- to 22-month-old) mice with established bone loss, activation of the INK-ATTAC caspase 8 in senescent cells or treatment with senolytics or the JAKi for 2–4 months resulted in higher bone mass and strength and better bone microarchitecture than in vehicle-treated mice. The beneficial effects of _targeting senescent cells were due to lower bone resorption with either maintained (trabecular) or higher (cortical) bone formation as compared to vehicle-treated mice. In vitro studies demonstrated that senescent-cell conditioned medium impaired osteoblast mineralization and enhanced osteoclast-progenitor survival, leading to increased osteoclastogenesis. Collectively, these data establish a causal role for senescent cells in bone loss with aging, and demonstrate that _targeting these cells has both anti-resorptive and anabolic effects on bone. Given that eliminating senescent cells and/or inhibiting their proinflammatory secretome also improves cardiovascular function4, enhances insulin sensitivity3, and reduces frailty7, _targeting this fundamental mechanism to prevent age-related bone loss suggests a novel treatment strategy not only for osteoporosis, but also for multiple age-related comorbidities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Baker, D.J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Xu, M. et al. _targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4, e12997 (2015).
Roos, C.M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Zhu, Y. et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Kirkland, J.L. & Tchkonia, T. Clinical strategies and animal models for developing senolytic agents. Exp. Gerontol. 68, 19–25 (2015).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 112, E6301–E6310 (2015).
LeBrasseur, N.K., Tchkonia, T. & Kirkland, J.L. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr. Inst. Workshop Ser. 83, 11–18 (2015).
Swanson, E.C., Manning, B., Zhang, H. & Lawrence, J.B. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J. Cell Biol. 203, 929–942 (2013).
Zhu, Y., Armstrong, J.L., Tchkonia, T. & Kirkland, J.L. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr. Opin. Clin. Nutr. Metab. Care 17, 324–328 (2014).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Jurk, D. et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11, 996–1004 (2012).
Jurk, D. et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 5, 4172 (2014).
Minamino, T. et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15, 1082–1087 (2009).
Farr, J.N. et al. Identification of senescent cells in the bone microenvironment. J. Bone Miner. Res. 31, 1920–1929 (2016).
Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55, 2284–2292 (1995).
Nelson, G. et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349 (2012).
Coppé, J.P., Desprez, P.Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Acosta, J.C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Herbig, U., Ferreira, M., Condel, L., Carey, D. & Sedivy, J.M. Cellular senescence in aging primates. Science 311, 1257 (2006).
Hamrick, M.W. et al. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone 39, 845–853 (2006).
Glatt, V., Canalis, E., Stadmeyer, L. & Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 22, 1197–1207 (2007).
Silva, M.J., Brodt, M.D. & Uthgenannt, B.A. Morphological and mechanical properties of caudal vertebrae in the SAMP6 mouse model of senile osteoporosis. Bone 35, 425–431 (2004).
Oliver, W.C. & Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564–1583 (1992).
McGee-Lawrence, M.E. et al. Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone 52, 296–307 (2013).
Qing, H. et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 27, 1018–1029 (2012).
Yi, J.-S. et al. Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI Insight 1, e90220 (2016).
D'Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 106, 256–271 (2015).
Arai, F. et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 190, 1741–1754 (1999).
Bellido, T. et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J. Clin. Invest. 95, 2886–2895 (1995).
Bendre, M.S. et al. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33, 28–37 (2003).
Daci, E., Verstuyf, A., Moermans, K., Bouillon, R. & Carmeliet, G. Mice lacking the plasminogen activator inhibitor 1 are protected from trabecular bone loss induced by estrogen deficiency. J. Bone Miner. Res. 15, 1510–1516 (2000).
Khosla, S. Odanacatib: location and timing are everything. J. Bone Miner. Res. 27, 506–508 (2012).
Mullard, A. Merck & Co. drops osteoporosis drug odanacatib. Nat. Rev. Drug Discov. 15, 669 (2016).
Lyles, K.W. et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N. Engl. J. Med. 357, 1799–1809 (2007).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, _targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Wright, N.C. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 29, 2520–2526 (2014).
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Miner. Res. 22, 465–475 (2007).
Stern, A.R. et al. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques 52, 361–373 (2012).
Lee, B.Y. et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 5, 187–195 (2006).
Syed, F.A. et al. Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J. Bone Miner. Res. 20, 1992–2001 (2005).
Tchkonia, T. et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am. J. Physiol. Endocrinol. Metab. 288, E267–E277 (2005).
Takeshita, S., Kaji, K. & Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488 (2000).
Gingery, A., Bradley, E., Shaw, A. & Oursler, M.J. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J. Cell. Biochem. 89, 165–179 (2003).
Acknowledgements
This work was supported by NIH grants P01 AG004875 (S.K.), R01 AG048792 (S.K.), K01 AR070241 (J.N.F.), K01 AR070281 (M.M.W.), R01 AR068275 (D.G.M.), R37 AG013925 (J.L.K.), AG R21 049182 (J.L.K.), the Connor Group, the Noaber, and the Ted Nash Foundations (J.L.K.), the Glenn Foundation (J.L.K., N.K.L.), and both a High-Risk Pilot Award (J.N.F. and S.K.) and Career Development Awards (J.N.F. and M.M.W.) from the Mayo Clinic Robert and Arlene Kogod Center on Aging, as well as the Richard F. Emslander Career Development Award in Endocrinology (J.N.F.), the James A. Ruppe Career Development Award in Endocrinology (M.M.W.), and the Glenn/American Federation for Aging Research Postdoctoral Fellowship for Translational Research on Aging (M.X.). We thank M. Ruan, G.L. Evans, B.S. Thicke, and J.M. Peterson (Mayo Clinic) for their technical assistance. We also thank A.R. Thoreson, A.W. Hooke (Mayo Clinic), and the Mayo Clinic Materials and Structural Testing Resource Laboratory for performing the bone biomechanical compression and nano-indentation testing.
Author information
Authors and Affiliations
Contributions
J.N.F. performed most of the experiments and analyses on INK-ATTAC and D + Q–treated mice. M.X. generated conditioned medium and defined the SASP mechanism. M.M.W. performed osteoclast cell culture experiments. M.X. and M.M.W. performed most of the experiments and analyses on JAKi-treated mice. D.G.M. provided technical guidance. D.G.F., J.L.O., B.A.N., J.G.S., M.B.O., C.M.H., T.P., T.T., N.K.L., M.T.D., R.J.P., and M.J.O. assisted with various aspects of the experiments and analyses. J.N.F., M.X., M.M.W., T.T., J.L.K., and S.K. contributed to the design of experiments. J.N.F., M.W., and S.K. wrote the manuscript with input from all co-authors. S.K. directed and supervised all aspects of the study in collaboration with J.L.K. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.L.K., T.T., and T.P. have a financial interest related to this research. A patent on senolytic drugs (WO2015116735A1) is held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Supplementary information
Supplementary Figures
Supplementary Figures 1–15. (PDF 1704 kb)
Rights and permissions
About this article
Cite this article
Farr, J., Xu, M., Weivoda, M. et al. _targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23, 1072–1079 (2017). https://doi.org/10.1038/nm.4385
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4385