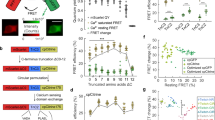
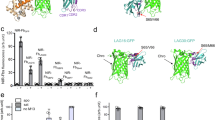
Abstract
The quality of genetically encoded calcium indicators (GECIs) has improved dramatically in recent years, but high-performing ratiometric indicators are still rare. Here we describe a series of fluorescence resonance energy transfer (FRET)-based calcium biosensors with a reduced number of calcium binding sites per sensor. These 'Twitch' sensors are based on the C-terminal domain of Opsanus troponin C. Their FRET responses were optimized by a large-scale functional screen in bacterial colonies, refined by a secondary screen in rat hippocampal neuron cultures. We tested the in vivo performance of the most sensitive variants in the brain and lymph nodes of mice. The sensitivity of the Twitch sensors matched that of synthetic calcium dyes and allowed visualization of tonic action potential firing in neurons and high resolution functional tracking of T lymphocytes. Given their ratiometric readout, their brightness, large dynamic range and linear response properties, Twitch sensors represent versatile tools for neuroscience and immunology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Palmer, A.E., Quin, Y., Park, J.G. & McCombs, J.E. Design and application of genetically encoded biosensors. Trends Biotechnol. 29, 144–152 (2011).
Looger, L.L. & Griesbeck, O. Genetically encoded neural activity indicators. Curr. Opin. Neurobiol. 22, 18–23 (2012).
Knöpfel, T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 13, 687–700 (2012).
Mank, M. & Griesbeck, O. Genetically encoded calcium indicators. Chem. Rev. 108, 1550–1564 (2008).
Miyawaki, A. et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Heim, N. & Griesbeck, O. Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. J. Biol. Chem. 279, 14280–14286 (2004).
Wang, Q., Shui, B., Kotlikoff, M.I. & Sondermann, H. Structural basis for calcium sensing by GCaMP2. Structure 16, 1817–1827 (2008).
Akerboom, J. et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J. Biol. Chem. 284, 6455–6464 (2009).
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141 (2001).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012).
Zariwala, H.A. et al. A Cre-cependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141 (2012).
Ohkura, M. et al. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS ONE 7, e51286 (2012).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011).
Nagai, T., Yamada, S., Tominaga, T., Ichikawa, M. & Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 101, 10554–10559 (2004).
Lütcke, H. et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Front. Neural Circuits 4, 9 (2010).
Palmer, A.E. et al. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem. Biol. 13, 521–530 (2006).
Horikawa, K. et al. Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat. Methods 7, 729–732 (2010).
Mank, M. et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods 5, 805–811 (2008).
Homma, R. et al. In vivo functional properties of juxtaglomerular neurons in the mouse olfactory bulb. Front. Neural Circuits 7, 23 (2013).
Kuchibhotla, K.V. et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59, 214–225 (2008).
Siffrin, V. et al. In vivo imaging of partially reversible Th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33, 424–436 (2010).
Vassylyev, D.G. et al. Crystal structure of troponin C in complex with troponin I fragment at 2.3-angstrom resolution. Proc. Natl. Acad. Sci. USA 95, 4847–4852 (1998).
Gordon, A.M., Homsher, E. & Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 80, 853–924 (2000).
Direnberger, S. et al. Biocompatibility of a genetically encoded calcium indicator in a transgenic mouse model. Nat. Commun. 3, 1031 (2012).
DeMaria, C.D., Soong, T.W., Alseikhan, B.A., Alvania, R.S. & Yue, D.T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411, 484–489 (2001).
Mank, M. et al. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys. J. 90, 1790–1796 (2006).
Wishart, D.S. & Case, D.A. Use of chemical shifts in macromolecular structure determination. Methods Enzymol. 338, 3–34 (2001).
Slupsky, C.M. & Sykes, B.D. NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry 34, 15953–15964 (1995).
Huang, J.R. & Grzesiek, S. Ensemble calculations of unstructured proteins constrained by RDC and PRE data: a case study of urea-denatured ubiquitin. J. Am. Chem. Soc. 132, 694–705 (2010).
Petoukhov, M.V. et al. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Cryst. 45, 342–350 (2012).
Geiger, A. et al. Correlating calcium binding, Förster resonance energy transfer, and conformational change in the biosensor TN-XXL. Biophys. J. 102, 2401–2410 (2012).
Markwardt, M.L. et al. An improved cerulean fluorescent protein with enhanced brightness and reduced reversible photoswitching. PLoS ONE 6, e17896 (2011).
Goedhart, J. et al. Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat. Methods 7, 137–139 (2010).
Mues, M. et al. Real-time in vivo analysis of T cell activation in the central nervous system using a genetically encoded calcium indicator. Nat. Med. 19, 778–783 (2013).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Bousso, P. & Moreau, H.D. Functional immunoimaging: the revolution continues. Nat. Rev. Immunol. 12, 858–864 (2012).
Feske, S., Skolnik, E.Y. & Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 12, 532–547 (2012).
Barnden, M.J. et al. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Ikura, M., Kay, L.E. & Bax, A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry 29, 4659–4667 (1990).
Grzesiek, S. & Bax, A. An efficient experiment for sequential backbone assignment of medium sized isotopically enriched proteins. J. Magn. Reson. 99, 201–207 (1992).
Grzesiek, S. & Bax, A. Correlating backbone amide and sidechain resonances in larger proteins by multiple relayed triple resonance NMR. J. Am. Chem. Soc. 114, 6291–6293 (1992).
Vuister, G.W. & Bax, A. Quantitative J correlation: a new approach for measuring homonuclear three bond J(HNHa) coupling constants in 15N-enriched proteins. J. Am. Chem. Soc. 115, 7772–7777 (1993).
Hansen, M.R., Mueller, L. & Pardi, A. Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions. Nat. Struct. Biol. 5, 1065–1074 (1998).
Ottiger, N., Delaglio, F. & Bax, A. Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J. Magn. Reson. 131, 373–378 (1998).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Keller, R.L.J. The Computer Aided Resonance Assignment Tutorial (Cantina, 2004).
Shen, Y. et al. Consistent blind protein structure generation from NMR chemical shift data. Proc. Natl. Acad. Sci. USA 105, 4685–4690 (2008).
Raman, S. et al. NMR structure determination for larger proteins using backbone-only data. Science 327, 1014–1018 (2010).
Koradi, R., Billeter, M. & Wuthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 (1996).
Pettersen, E.F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Laskowski, R.A., Rullmannn, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 (1996).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Konarev, P.V., Petoukhov, M.V., Volkov, V.V. & Svergun, D.I. ATSAS 2.1, a program package for small-angle scattering data analysis. J. Appl. Cryst. 39, 277–286 (2006).
Putnam, C.D., Hammel, M., Hura, G.L. & Tainer, J.A. X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 40, 191–285 (2007).
Svergun, D.I., Petoukhov, M.V. & Koch, M.H.J. Determination of domain structure of proteins from X-ray solution scattering. Biophys. J. 80, 2946–2953 (2001).
Volkov, V.V. & Svergun, D.I. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Cryst. 36, 860–864 (2003).
Petoukhov, M.V. et al. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Cryst. 45, 342–350 (2012).
Wriggers, W. & Chacon, P. Using Situs for the registration of protein structures with low-resolution bead models from X-ray solution scattering. J. Appl. Cryst. 34, 773–776 (2001).
Tsien, R. & Pozzan, T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 172, 230–262 (1989).
Wardill, T.J. et al. A neuron-based screening platform for optimizing genetically encoded calcium indicators. PLoS ONE 8, e77728 (2013).
Niell, C.M. & Stryker, M.P. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 28, 7520–7536 (2008).
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. USA 100, 7319–7324 (2003).
Pologruto, T.A., Yasuda, R. & Svoboda, K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J. Neurosci. 24, 9572–9579 (2004).
Kerlin, A.M., Andermann, M.L., Berezovskii, V.K. & Reid, R.C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010).
Brainard, D.H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Pelli, D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Ohki, K., Chung, S., Ch'Ng, Y.H., Kara, P. & Reid, R.C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Garaschuk, O., Hanse, E. & Konnerth, A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. (Lond.) 507, 219–236 (1998).
Acknowledgements
This work was funded by the Max Planck Society, the Howard Hughes Medical Institute, Deutsche Forschungsgemeinschaft (DFG) grant SFB 870 (to O. Griesbeck), DFG grant GRK1721 (to G.W.), EU FP7 EuroV1sion grant (to O. Griesbeck), US National Science Foundation grant IBN-9985315 (to T.A.), US National Science Foundation grant IOS 1145981 (to L.C.R.) and the DFG Center for Integrative Neuroscience (to O. Garaschuk).
Author information
Authors and Affiliations
Contributions
T.T. characterized the minimal domain, cloned constructs and performed protein purifications and in vitro spectroscopic characterizations; J.L. established the bacterial colony screen and performed colony screening and further protein purifications; M.M. and I.B. performed in vivo imaging of T lymphocytes; L.R., S.B., Y. Laukat and C.G. performed NMR structure determination and interpreted results; T.A. and L.C.R. cloned toadfish TnC; A.G. and T.T. collected SAXS data; G.W. calculated SAXS models; H.D. performed in vivo characterization in mouse visual cortex; Y.K., Y. Liang, G.K. and O. Garaschuk planned, performed and interpreted characterization of the sensors in cortical slices in situ and mouse olfactory bulb in vivo; T.T., T.W.C., H.D. and D.S.K. planned, performed and interpreted neuronal screening results. O. Griesbeck designed experiments, supervised sensor engineering and screening and integrated results from the collaborators. T.T., H.D., C.G., O. Garaschuk and O. Griesbeck wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1–3 (PDF 2179 kb)
Rights and permissions
About this article
Cite this article
Thestrup, T., Litzlbauer, J., Bartholomäus, I. et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat Methods 11, 175–182 (2014). https://doi.org/10.1038/nmeth.2773
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2773
This article is cited by
-
Angiotensin receptors and α1B-adrenergic receptors regulate native IK(ACh) and phosphorylation-deficient GIRK4 (S418A) channels through different PKC isoforms
Pflügers Archiv - European Journal of Physiology (2024)
-
Fluorescent sensors for imaging of interstitial calcium
Nature Communications (2023)
-
Subcellular second messenger networks drive distinct repellent-induced axon behaviors
Nature Communications (2023)
-
Advances in NK cell therapy for brain tumors
npj Precision Oncology (2023)
-
A general method for the development of multicolor biosensors with large dynamic ranges
Nature Chemical Biology (2023)