Key Points
-
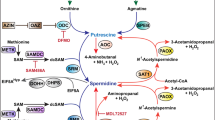
Polyamines are naturally occurring organic cations found in plants, animals and microbes. They are formed by the enzymatic decarboxylation of the amino acids ornithine or arginine.
-
Ornithine decarboxylase (ODC) is the first enzyme in the polyamine synthesis pathway in mammals and is the _target for difluoromethylornithine (DFMO), a substrate analogue and specific inhibitor that irreversibly inactivates ODC when it binds to the active site of the enzyme.
-
ODC and several other polyamine metabolic proteins are essential for normal cell and tissue functions, including growth, development and tissue repair. ODC and polyamine content are increased in many cancers arising from epithelial tissues, such as the skin and colon.
-
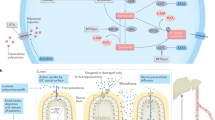
Polyamines exert their effects in eukaryotic cells in part by regulating specific gene expression.
-
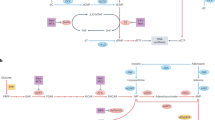
In murine and human colonic mucosal tissue, ODC is negatively regulated by the adenomatous polyposis coli (APC) tumour-suppressor gene. APC is mutated or deleted in the germline of people with familial adenomatous polyposis (FAP), a genetic syndrome associated with a high risk of colon cancer. APC is also mutated or deleted in somatic colon epithelial cells in most sporadic, or non-genetic, forms of colon cancer.
-
Loss of APC function causes an increase in ODC activity and polyamine biosynthesis, and tumour formation in ApcMin/+ mice, a murine model of human FAP. Treatment of ApcMin/+ mice with DFMO suppresses intestinal tumour formation.
-
Several non-steroidal anti-inflammatory drugs (NSAIDs), the use of which is associated with decreased risk of epithelial cancers, activate the transcription of spermidine/spermine N1-acetyltransferase, the first enzyme in the polyamine catabolic pathway. Experimental studies indicate that combinations of DFMO and NSAIDs are potent inhibitors of colon and intestinal cancer development in murine models.
-
Clinical studies have shown that DFMO is well tolerated and can prevent the development of precancerous lesions in the skin. Several large randomized trials involving the skin, colon and other organ sites are underway.
Abstract
The amino-acid-derived polyamines have long been associated with cell growth and cancer, and specific oncogenes and tumour-suppressor genes regulate polyamine metabolism. Inhibition of polyamine synthesis has proven to be generally ineffective as an anticancer strategy in clinical trials, but it is a potent cancer chemoprevention strategy in preclinical studies. Clinical trials, with well-defined goals, are now underway to evaluate the chemopreventive efficacy of inhibitors of polyamine synthesis in a range of tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cohen, S. S. A Guide to the Polyamines. (Oxford Univ. Press, New York, 1998).
Morris, S. M. Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 22, 87–105 (2002).
Bardocz, S. et al. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 73, 819–828 (1995).
Milovic, V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur. J. Gastroenterol. Hepatol. 13, 1021–1025 (2001).
Tabor, H., Hafner, E. W. & Tabor, C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J. Bacteriol. 144, 952–956 (1980).
Tabor, C. W., Tabor, H., Tyagi, A. K. & Cohn, M. S. The biochemistry, genetics, and regulation of polyamine biosynthesis in Saccharomyces cerevisiae. Fed. Proc. 41, 3084–3088 (1982).
Pendeville, H. et al. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 21, 6549–6558 (2001). Showed that ODC is an essential gene in mammals and that lack of ODC function resulted in increased apoptosis in developing embryos.
Chang, Z. F. & Chen, K. Y. Regulation of ornithine decarboxylase and other cell cycle-dependent genes during senescence of IMR-90 human diploid fibroblasts. J. Biol. Chem. 263, 11431–11435 (1988).
Gerner, E. W., Garewal, H. S., Emerson, S. S. & Sampliner, R. E. Gastrointestinal tissue polyamine contents of patients with Barrett's esophagus treated with α-difluoromethylornithine. Cancer Epidemiol. Biomarkers Prev. 3, 325–330 (1994).
Russell, D. & Snyder, S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl Acad. Sci. USA 60, 1420–1427 (1968).
Andersson, G. & Heby, O. Polyamine and nucleic acid concentrations in Ehrlich ascites carcinoma cells and liver of tumor-bearing mice at various stages of tumor growth. J. Natl Cancer Inst. 48, 165–172 (1972).
Wallace, H. M. & Caslake, R. Polyamines and colon cancer. Eur. J. Gastroenterol. Hepatol. 13, 1033–1039 (2001).
O'Brien, T. G., Simsiman, R. C. & Boutwell, R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 35, 1662–1670 (1975).
Ahmad, N., Gilliam, A. C., Katiyar, S. K., O'Brien, T. G. & Mukhtar, H. A definitive role of ornithine decarboxylase in photocarcinogenesis. Am. J. Pathol. 159, 885–892 (2001).
Marsh, J. P. & Mossman, B. T. Role of asbestos and active oxygen species in activation and expression of ornithine decarboxylase in hamster tracheal epithelial cells. Cancer Res. 51, 167–173 (1991).
Crozat, A., Palvimo, J. J., Julkunen, M. & Janne, O. A. Comparison of androgen regulation of ornithine decarboxylase and S-adenosylmethionine decarboxylase gene expression in rodent kidney and accessory sex organs. Endocrinology 130, 1131–1144 (1992).
Mohan, R. R. et al. Overexpression of ornithine decarboxylase in prostate cancer and prostatic fluid in humans. Clin. Cancer Res. 5, 143–147 (1999).
Meyskens, F. L. Jr. & Gerner, E. W. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin. Cancer Res. 5, 945–951 (1999).
Thomas, T., Faaland, C. A., Adhikarakunnathu, S. & Thomas, T. J. Structure-activity relations of S-adenosylmethionine decarboxylase inhibitors on the growth of MCF-7 breast cancer cells. Breast Cancer Res. Treat. 39, 293–306 (1996).
Casero, R. A. Jr. et al. The role of polyamine catabolism in anti-tumour drug response. Biochem. Soc. Trans. 31, 361–365 (2003).
Eskens, F. A. et al. Phase I and pharmacological study of weekly administration of the polyamine synthesis inhibitor SAM 486A (CGP 48 664) in patients with solid tumors. European Organization for Research and Treatment of Cancer Early Clinical Studies Group. Clin. Cancer Res. 6, 1736–1743 (2000).
Wolff, A. C. et al. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 9, 5922–5928 (2003).
Giardiello, F. M. et al. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 57, 199–201 (1997). Showed that colonic ODC activity and polyamine levels were increased in patients with FAP, a genetic form of colon cancer.
Groden, J. et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66, 589–600 (1991).
Kinzler, K. W. et al. Identification of FAP locus genes from chromosome 5q21. Science 253, 661–665 (1991).
Iwamoto, M., Ahnen, D. J., Franklin, W. A. & Maltzman, T. H. Expression of β-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis 21, 1935–1940 (2000).
He, T. C. et al. (1998) Identification of c-MYC as a _target of the APC pathway. Science 281, 1509–1512.
Boyd, K. E. & Farnham, P. J. Identification of _target genes of oncogenic transcription factors. Proc. Soc. Exp. Biol. Med. 222, 9–28 (1999).
Hermeking, H. The MYC oncogene as a cancer drug _target. Curr. Cancer Drug _targets 3, 163–175 (2003).
Bello-Fernandez, C., Packham, G. & Cleveland, J. L. The ornithine decarboxylase gene is a transcriptional _target of c-Myc. Proc. Natl Acad. Sci. USA 90, 7804–7808 (1993). Showed that ODC is a transcriptional _target of the MYC oncogene
Pena, A. et al. Regulation of human ornithine decarboxylase expression by the c-Myc.Max protein complex. J. Biol. Chem. 268, 27277–27285 (1993).
Erdman, S. H. et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis 20, 1709–1713 (1999). Demonstrated that ODC RNA was upregulated, OAZ RNA was downregulated and intestinal polyamine content increased as a consequence of loss of wild-type APC in a mouse model.
Fultz, K. E. & Gerner, E. W. APC-dependent regulation of ornithine decarboxylase in human colon tumor cells. Mol. Carcinog. 34, 10–18 (2002).
Shantz, L. M. & Pegg, A. E. Ornithine decarboxylase induction in transformation by H-Ras and RhoA. Cancer Res. 58, 2748–2753 (1998).
Smith, M. K., Trempus, C. S. & Gilmour, S. K. Co-operation between follicular ornithine decarboxylase and v-Ha-ras induces spontaneous papillomas and malignant conversion in transgenic skin. Carcinogenesis 19, 1409–1415 (1998).
Ignatenko, N. A., Babbar, N., Mehta, D., Casero, R. A. Jr & Gerner, E. W. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol. Carcinog. 39, 91–102 (2004).
Babbar, N., Ignatenko, N. A., Casero, R. A. Jr & Gerner, E. W. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J. Biol. Chem. 278, 47762–47775 (2003). Established SSAT as a transcriptional _target of the PPARγ tumour suppressor and showed that the sulphone metabolite of the NSAID sulindac induced SSAT transcription by activating PPARγ.
Bai, G. et al. Androgen regulation of the human ornithine decarboxylase promoter in prostate cancer cells. J. Androl. 19, 127–135 (1998).
De Benedetti, A. & Harris, A. L. eIF4E expression in tumors: its possible role in progression of malignancies. Int. J. Biochem. Cell Biol. 31, 59–72 (1999).
Martinez, M. E. et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc. Natl Acad. Sci. USA 100, 7859–7864 (2003). Showed that an SNP in the ODC promoter was associated, with reduced risk of colon-polyp recurrence in people reporting aspirin use. Provided evidence that the ODC SNP reduced polyamine synthesis, while aspirin induced polyamine catabolism and export, thereby reducing polyamine levels.
Ignatenko, N. A. et al. The chemopreventive agent α-difluoromethylornithine blocks K-ras dependent tumor formation and specific gene expression in Caco-2 cells. Mol. Carcinog. 39, 221–233 (2004).
Brzozowski, T., Konturek, S. J., Drozdowicz, D., Dembinski, A. & Stachura, J. Healing of chronic gastric ulcerations by L-arginine. Role of nitric oxide, prostaglandins, gastrin and polyamines. Digestion. 56, 463–471 (1995).
Xie, X., Tome, M. E. & Gerner, E. W. Loss of intracellular putrescine pool-size regulation induces apoptosis. Exp. Cell Res. 230, 386–392 (1997).
Erez, O., Goldstaub, D., Friedman, J. & Kahana, C. Putrescine activates oxidative stress dependent apoptotic death in ornithine decarboxylase overproducing mouse myeloma cells. Exp. Cell Res. 281, 148–156 (2002).
Takigawa, M. et al. Tumor angiogenesis and polyamines: α-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits B16 melanoma-induced angiogenesis in ovo and the proliferation of vascular endothelial cells in vitro. Cancer Res. 50, 4131–4138 (1990).
Takahashi, Y., Mai, M. & Nishioka, K. α-difluoromethylornithine induces apoptosis as well as anti-angiogenesis in the inhibition of tumor growth and metastasis in a human gastric cancer model. Int. J. Cancer 85, 243–247 (2000).
Bauer, P. M., Buga, G. M., Fukuto, J. M., Pegg, A. E. & Ignarro, L. J. Nitric oxide inhibits ornithine decarboxylase via S-nitrosylation of cysteine 360 in the active site of the enzyme. J. Biol. Chem. 276, 34458–34464 (2001).
Buga, G. M., Wei, L. H., Bauer, P. M., Fukuto, J. M. & Ignarro, L. J. (1998) NG-hydroxy-L-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. 275, R1256–R1264.
Itoh, M. & Bissell, M. J. The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis. J. Mammary Gland Biol. Neoplasia 8, 449–462 (2003).
Trosko, J. E. The role of stem cells and gap junctional intercellular communication in carcinogenesis. J. Biochem. Mol. Biol. 36, 43–48 (2003).
Guo, X. et al. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am. J. Physiol. Cell Physiol. 285, C1174–C1187 (2003).
Shore, L., McLean, P., Gilmour, S. K., Hodgins, M. B. & Finbow, M. E. Polyamines regulate gap junction communication in connexin 43-expressing cells. Biochem. J. 357, 489–495 (2001).
Pegg, A. E. et al. Transgenic mouse models for studies of the role of polyamines in normal, hypertrophic and neoplastic growth. Biochem. Soc. Trans. 31, 356–360 (2003).
Williams-Ashman, H. G. & Schenone, A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem. Biophys. Res. Commun. 46, 288–295 (1972).
Mamont, P. S. et al. α-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc. Natl Acad. Sci. USA 73, 1626–1630 (1976).
Bey, P. et al. Analogues of ornithine as inhibitors of ornithine decarboxylase. New deductions concerning the topography of the enzyme's active site. J. Med. Chem. 21, 50–55 (1978).
Doua, F. & Yapo, F. B. Human trypanosomiasis in the Ivory Coast: therapy and problems. Acta Trop. 54, 163–168 (1993).
Seiler, N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr. Drug _targets 4, 537–564 (2003).
Bitonti, A. J. et al. Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J. Biol. Chem. 265, 382–388 (1990).
Seiler, N., Duranton, B. & Raul, F. The polyamine oxidase inactivator MDL 72527. Prog. Drug Res. 59, 1–40 (2002).
Mamont, P. S., Claverie, N. & Gerhart, F. Fluorine-containing polyamines: biochemistry and potential applications. Adv. Exp. Med. Biol. 250, 691–706 (1988).
McCann, P. P. & Pegg, A. E. Ornithine decarboxylase as an enzyme _target for therapy. Pharmacol. Ther. 54, 195–215 (1992).
McCann, P. P., Pegg, A. E. & Sjoerdsma, A. Inhibition of Polyamine Metabolism, Biological Significance and Basis for New Therapies (Academic, Orlando, 1987).
Lawson, K. R., Ignatenko, N. A., Piazza, G. A., Cui, H. & Gerner, E. W. Influence of K-ras activation on the survival responses of Caco-2 cells to the chemopreventive agents sulindac and difluoromethylornithine. Cancer Epidemiol. Biomarkers Prev. 9, 1155–1162 (2000).
Weeks, C. E., Herrmann, A. L., Nelson, F. R. & Slaga, T. J. α-Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits tumor promoter-induced polyamine accumulation and carcinogenesis in mouse skin. Proc. Natl Acad. Sci. USA 79, 6028–6032 (1982). Demonstrated that DFMO inhibited chemically induced skin carcinogenesis by a mechanism affecting tumour promotion. Provided the first evidence from animal models supporting the rationale for DFMO as a chemopreventive agent.
Meyskens, F. L. Jr et al. Effect of α-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J. Natl Cancer Inst. 90, 1212–1218 (1998). Demonstrated the safety of DFMO and its potent biochemical effect on polyamine content in human colon tissue over a 1-year period; a critical study for planning of Phase III trials.
Rao, C. V., Tokumo, K., Rigotty, J., Zang, E., Kelloff, G. & Reddy, B. S. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, α-difluoromethylornithine, 16 α-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 51, 4528–4534 (1991).
Croghan, M. K., Aickin, M. G. & Meyskens, F. L. Dose-related α-difluoromethylornithine ototoxicity. Am. J. Clin. Oncol. 14, 331–335 (1991).
Pasic, T. R., Heisey, D. & Love, R. R. α-difluoromethylornithine ototoxicity. Chemoprevention clinical trial results. Arch. Otolaryngol. Head Neck Surg. 123, 1281–1286 (1997).
Doyle, K. J., McLaren, C. E., Shanks, J. E., Galus, C. M. & Meyskens, F. L. Effects of difluoromethylornithine chemoprevention on audiometry thresholds and otoacoustic emissions. Arch. Otolaryngol. Head Neck Surg. 127, 553–558 (2001).
Mitchell, M. F. et al. Phase I dose de-escalation trial of α-difluoromethylornithine in patients with grade 3 cervical intraepithelial neoplasia. Clin. Cancer Res. 4, 303–310 (1998).
Carbone, P. P. et al. Phase I chemoprevention study of difluoromethylornithine in subjects with organ transplants. Cancer Epidemiol. Biomarkers Prev. 10, 657–661 (2001).
Love, R. R. et al. Randomized phase I chemoprevention dose-seeking study of α-difluoromethylornithine. J. Natl Cancer Inst. 85, 732–737 (1993).
Alberts, D. S. et al. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol. Biomarkers Prev. 9, 1281–1286 (2000). Clinical study showing efficacy of DFMO in the treatment of actinic keratoses in humans.
Boyle, J. O., Meyskens, F. L. Jr, Garewal, H. S. & Gerner, E. W. Polyamine contents in rectal and buccal mucosae in humans treated with oral difluoromethylornithine. Cancer Epidemiol. Biomarkers Prev. 1, 131–135 (1992).
Simoneau, A. R., Gerner, E. W., Phung, M., McLaren, C. E. & Meyskens, F. L. Jr. α-difluoromethylornithine and polyamine levels in the human prostate: results of a phase IIa trial. J. Natl Cancer Inst. 93, 57–59 (2001).
Fabian, C. J. et al. A phase II breast cancer chemoprevention trial of oral α-difluoromethylornithine: breast tissue, imaging, and serum and urine biomarkers. Clin. Cancer Res. 8, 3105–3117 (2002).
Meyskens, F. L. Jr et al. Dose de-escalation chemoprevention trial of α-difluoromethylornithine in patients with colon polyps. J. Natl Cancer Inst. 86, 1122–1130 (1994). Clinical study that used a novel dose de-escalation design to identify the lowest does of DFMO that still caused the desired biochemical effect (polyamine depletion) in the _target tissue.
Levy, G. N. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. FASEB J. 11, 234–247 (1997).
Oshima, M. et al. Suppression of intestinal polyposis in Apc δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell, 87, 803–809 (1996).
Phillips, R. K. et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 50, 857–860 (2002).
Bishop-Bailey, D., Calatayud, S., Warner, T. D., Hla, T. & Mitchell, J. A. Prostaglandins and the regulation of tumor growth. J. Environ. Pathol. Toxicol. Oncol. 21, 93–101 (2002).
Hughes, A., Smith, N. I. & Wallace, H. M. Polyamines reverse non-steroidal anti-inflammatory drug-induced toxicity in human colorectal cancer cells. Biochem. J. 374, 481–488 (2003).
Jacoby, R. F. et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 60, 1864–1870 (2000).
Meyskens, F. L. Jr et al. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J. Natl Cancer Inst. 86, 539–543 (1994).
Hong, W. K. et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 323, 795–801 (1990).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 90, 1371–1388 (1998).
Mitchell, J. L., Hoff, J. A. & Bareyal-Leyser, A. Stable ornithine decarboxylase in a rat hepatoma cell line selected for resistance to α-difluoromethylornithine. Arch. Biochem. Biophys. 290, 143–152 (1991).
Meyskens, F. L. Jr & Szabo, E. How should we move the field of chemoprevention agent development forward in a productive manner. Eur. J. Cancer (in the press).
Levin, V. A. et al. Phase III randomized study of postradiotherapy chemotherapy with combination α-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin. Cancer Res. 9, 981–990 (2003).
Lux, G. D., Marton, L. J. & Baylin, S. B. Ornithine decarboxylase is important in intestinal mucosal maturation and recovery from injury in rats. Science 210, 195–198 (1980). Showed the importance of polyamines in normal intestinal development and repair of damage.
Yarrington, J. T. et al. Intestinal changes caused by DL-α-difluoromethylornithine (DFMO), an inhibitor of ornithine decarboxylase. Exp. Mol. Pathol. 39, 300–316 (1983).
Wang, J. Y. & Johnson, L. R. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am. J. Physiol. 259, G584–G592 (1990).
Wang, J. Y. & Johnson, L. R. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 100, 333–343 (1991).
Babal, P., Manuel, S. M., Olson, J. W. & Gillespie, M. N. Cellular disposition of transported polyamines in hypoxic rat lung and pulmonary arteries. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L610–L617 (2000).
Ahuja, V., Tantry, U., Park, J. & Barbul, A. Effect of difluoromethylornitine,a chemotherapeutic agent, on wound healing. J. Surg. Res. 114, 308–309 (2003).
Calandra, R. S., Rulli, S. B., Frungieri, M. B., Suescun, M. O. & Gonzalez-Calvar, S. I. Polyamines in the male reproductive system. Acta Physiol. Pharmacol. Ther. Latinoam. 46, 209–222 (1996).
Guha, S. K. & Janne, J. Decarboxylation of ornithine and adenosymethionine in rat ovary during pregnancy. Acta Endocrinol. (Copenh.) 81, 793–800 (1976).
Hoshiai, H., Lin, Y. C., Loring, J. M., Perelle, B. A. & Villee, C. A. Ornithine decarboxylase activity and polyamine content of the placenta and decidual tissue in the rat. Placenta 2, 105–116 (1981).
Min, S. H. et al. Altered levels of growth-related and novel gene transcripts in reproductive and other tissues of female mice overexpressing spermidine/spermine N1-acetyltransferase (SSAT). J. Biol. Chem. 277, 3647–3657 (2002).
Humphreys, M. H., Etheredge, S. B., Lin, S. Y., Ribstein, J. & Marton, L. J. Renal ornithine decarboxylase activity, polyamines, and compensatory renal hypertrophy in the rat. Am. J. Physiol. 255, F270–F277 (1988).
Mackintosh, C. A., Feith, D. J., Shantz, L. M. & Pegg, A. E. Overexpression of antizyme in the hearts of transgenic mice prevents the isoprenaline-induced increase in cardiac ornithine decarboxylase activity and polyamines, but does not prevent cardiac hypertrophy. Biochem. J. 350, 645–653 (2000).
Park, M. H., Lee, Y. B. & Joe, Y. A. Hypusine is essential for eukaryotic cell proliferation. Biol. Signals 6, 115–123 (1997).
Schnier, J., Schwelberger, H. G., Smit-McBride, Z., Kang, H. A. & Hershey, J. W. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 3105–3114 (1991).
Kang, H. A. & Hershey, J. W. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J. Biol. Chem. 269, 3934–3940 (1994).
Tome, M. E., Fiser, S. M., Payne, C. M. & Gerner, E. W. Excess putrescine accumulation inhibits the formation of modified eukaryotic initiation factor 5A (eIF-5A) and induces apoptosis. Biochem. J. 328, 847–854 (1997).
Bevec, D. & Hauber, J. Eukaryotic initiation factor 5A activity and HIV-1 Rev function. Biol. Signals 6, 124–133 (1997).
Zuk, D. & Jacobson, A. A single amino acid substitution in yeast eIF-5A results in mRNA stabilization. EMBO J. 17, 2914–2925 (1998).
Hayashi, S., Murakami, Y. & Matsufuji, S. Ornithine decarboxylase antizyme: a novel type of regulatory protein. Trends Biochem. Sci. 21, 27–30 (1996).
Matsufuji, S., Matsufuji, T., Wills, N. M., Gesteland, R. F. & Atkins, J. F. Reading two bases twice: mammalian antizyme frameshifting in yeast. EMBO J. 15, 1360–1370 (1996).
Tsuji, T. et al. Induction of epithelial differentiation and DNA demethylation in hamster malignant oral keratinocyte by ornithine decarboxylase antizyme. Oncogene 20, 24–33 (2001).
Casero, R. A. Jr & Pegg, A. E. Spermidine/spermine N1-acetyltransferase: the turning point in polyamine metabolism. FASEB J. 7, 653–661 (1993).
Thomas, T. & Thomas, T. J. Polyamine metabolism and cancer. J. Cell. Mol. Med. 7, 113–126 (2003).
Eimer, S., Lakowski, B., Donhauser, R. & Baumeister, R. Loss of spr-5 bypasses the requirement for the C. elegans presenilin sel-12 by derepressing hop-1. EMBO J. 21, 5787–5796 (2002).
Hixson, L. J., Emerson, S. S., Shassetz, L. R. & Gerner, E. W. Sources of variability in estimating ornithine decarboxylase activity and polyamine contents in human colorectal mucosa. Cancer Epidemiol. Biomarkers Prev. 3, 317–323 (1994).
Xie, X., Gillies, R. J. & Gerner, E. W. Characterization of a diamine exporter in Chinese hamster ovary cells and identification of specific polyamine substrates. J. Biol. Chem. 272, 20484–20489 (1997).
Wang, Y. et al. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem. Biophys. Res. Commun. 304, 605–611 (2003).
Cornbleet, M. A. et al. Phase I study of methylacetylenic putrescine, an inhibitor of polyamine biosynthesis. Cancer Chemother. Pharmacol. 23, 348–352 (1989).
Gastaut, J. A. et al. Treatment of acute myeloid leukemia and blastic phase of chronic myeloid leukemia with combined eflornithine (α difluoromethylornithine) and methylglyoxal-bis-guanyl hydrazone (methyl-GAG). Cancer Chemother. Pharmacol. 20, 344–348 (1987).
Wilding, G. et al. Phase I trial of the polyamine analog N1,N14-diethylhomospermine (DEHSPM) in patients with advanced solid tumors. Invest. New Drugs 22, 131–138 (2004).
Thompson, I. M. et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 349, 215–224 (2003).
Acknowledgements
The authors thank K. Nicolini and M. L. Myers for editorial assistance. The work described in this review was supported in part by grants from the United States Public Health Service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Cancer.gov
Entrez Gene
OMIM
familial adenomatous polyposis
FURTHER INFORMATION
Arizona Cancer Center Specialized Program of Research Excellence
MD Anderson Cancer Center chemoprevention trial with DFMO
MD Anderson Cancer Center chemoprevention trial with DFMO and celecoxib
University of California, Irvine, chemoprevention trial with DFMO and sulindac
Glossary
- UREA CYCLE
-
The key metabolic pathway in mammals for eliminating cellular breakdown products containing nitrogen.
- PROTEASOMAL DEGRADATION
-
Degradation of proteins involving the proteasome, a 26S multiprotein complex that catalyses the breakdown of polyubiquitylated proteins. Ornithine decarboxylase is the only non-ubiquitylated protein known to be degraded by the 26S proteasome.
- SINGLE-NUCLEOTIDE POLYMORPHISMS
-
(SNPs). Single-base-pair changes in DNA that differ among individuals.
- COLON POLYP
-
Non-invasive but neoplastic growths that develop from normal colon mucosa and that can develop into colon cancer.
- TIGHT JUNCTIONS
-
Intercellular junctions that act as barriers to specific tissue processes.
- GAP JUNCTIONS
-
The most widespread type of intercellular junction, involved in coupling cells both electrically and metabolically.
- RIBOSOME
-
Particles composed of RNA and protein that are sites of protein synthesis.
- PURE TONE
-
A single frequency tone measured as part of clinical audiometric evaluations.
Rights and permissions
About this article
Cite this article
Gerner, E., Meyskens, F. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4, 781–792 (2004). https://doi.org/10.1038/nrc1454
Issue Date:
DOI: https://doi.org/10.1038/nrc1454
This article is cited by
-
A study on phytogenotoxicity induced by biogenic amines: cadaverine and putrescine
Environmental Science and Pollution Research (2024)
-
Triptolide attenuates irritable bowel syndrome via inhibiting ODC1
BMC Gastroenterology (2023)
-
Polyamines: their significance for maintaining health and contributing to diseases
Cell Communication and Signaling (2023)
-
Un_targeted metabolomics reveals alternations in metabolism of bovine mammary epithelial cells upon IFN-γ treatment
BMC Veterinary Research (2023)
-
Annotation-free discovery of functional groups in microbial communities
Nature Ecology & Evolution (2023)