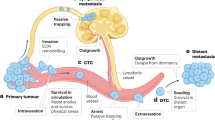
Abstract
An enduring problem in cancer research is the failure to reproduce highly encouraging preclinical therapeutic findings using transplanted or spontaneous primary tumours in mice in clinical trials of patients with advanced metastatic disease. There are several reasons for this, including the failure to model established, visceral metastatic disease. We therefore developed various models of aggressive multi-organ spontaneous metastasis after surgical resection of orthotopically transplanted human tumour xenografts. In this Opinion article we provide a personal perspective summarizing the prospect of their increased clinical relevance. This includes the reduced efficacy of certain _targeted anticancer drugs, the late emergence of spontaneous brain metastases and the clinical trial results evaluating a highly effective therapeutic strategy previously tested using such models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilmanns, C., Fan, D., O'Brian, C. A., Bucana, C. D. & Fidler, I. J. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int. J. Cancer 52, 98–104 (1992).
Kerbel, R. S. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 17, 301–304 (1998).
Kerbel, R. S. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived - but they can. be improved. Cancer Biol. Ther. 2, 108–113 (2003).
Peterson, J. K. & Houghton, P. J. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer 40, 837–844 (2004).
Van Dyke, T. & Jacks, T. Cancer modeling in the modern era: progress and challenges. Cell 108, 135–144 (2002).
Ottewell, P. D., Coleman, R. E. & Holen, I. From genetic abnormality to metastases: murine models of breast cancer and their use in the development of anticancer therapies. Breast Cancer Res. Treat. 96, 101–113 (2006).
Talmadge, J. E., Singh, R. K., Fidler, I. J. & Raz, A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol. 170, 793–804 (2007).
Brodie, S. G. et al. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene 20, 7514–7523 (2001).
Stambolic, V. et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten± mice. Cancer Res. 60, 3605–3611 (2000).
Gourley, C. et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J. Clin. Oncol. 28, 2505–2511 (2010).
Albiges, L. et al. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann. Oncol. 16, 1846–1847 (2005).
Schmitz, M. et al. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int. J. Cancer 120, 1284–1292 (2007).
Khanna, C. & Hunter, K. Modeling metastasis in vivo. Carcinogenesis 26, 513–523 (2005).
Sharpless, N. E. & DePinho, R. A. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Rev. Drug Discov. 5, 741–754 (2006).
Heyer, J., Kwong, L. N., Lowe, S. W. & Chin, L. Non-germline genetically engineered mouse models for translational cancer research. Nature Rev. Cancer 10, 470–480 (2010).
Kim, I. S. & Baek, S. H. Mouse models for breast cancer metastasis. Biochem. Biophys. Res. Commun. 394, 443–447 (2010).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Bergers, G., Song, S., Meyer-Morse, N., Bergsland, E. & Hanahan, D. Benefits of _targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111, 1287–1295 (2003).
Singh, M. et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nature Biotech. 28, 585–593 (2010).
Francia, G. & Kerbel, R. S. Raising the bar for cancer therapy models. Nature Biotech. 28, 561–562 (2010).
Van, D. T. Approximating a human cancer. Nature Med. 16, 976–977 (2010).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Morikawa, K., Walker, S. M., Jessup, J. M. & Fidler, I. J. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 48, 1943–1948 (1988).
Kubota, T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J. Cell. Biochem. 56, 4–8 (1994).
Furukawa, T., Kubota, T., Watanabe, M., Kitajima, M. & Hoffman, R. M. A novel “patient-like” treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 53, 3070–3072 (1993).
Kiguchi, K. et al. A patient-like orthotopic implantation nude mouse model of highly metastatic human ovarian cancer. Clin. Exp. Metastasis 16, 751–756 (1998).
Price, J. E. & Zhang, R. D. Studies of human breast cancer metastasis using nude mice. Cancer Metastasis Rev. 8, 285–297 (1990).
Fidler, I. J. Models for spontaneous metastasis. Cancer Res. 66, 9787 (2006).
Dawson, M. R., Duda, D. G., Fukumura, D. & Jain, R. K. VEGFR1-activity-independent metastasis formation. Nature 461, e4 (2009).
Nangia-Makker, P. et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl Cancer Inst. 94, 1854–1862 (2002).
Teicher, B. A. et al. Potentiation of cytotoxic cancer therapies by TNP-470 alone and with other anti-angiogenic agents. Int. J. Cancer 57, 920–925 (1994).
Holden, S. A., Emi, Y., Kakeji, Y., Northey, D. & Teicher, B. A. Host distribution and response to antitumor alkylating agents of EMT-6 tumor cells from subcutaneous tumor implants. Cancer Chemother. Pharmacol. 40, 87–93 (1997).
Barnett, S. C. & Eccles, S. A. Studies of mammary carcinoma metastasis in a mouse model system. II: lectin binding properties of cells in relation to the incidence and organ distribution of metastases. Clin. Exp. Metastasis 2, 297–310 (1984).
Munoz, R. et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 66, 3386–3391 (2006).
Cruz-Munoz, W., Man, S., Xu, P. & Kerbel, R. S. Development of a preclinical model of spontaneous human melanoma CNS metastasis. Cancer Res. 68, 4500–4505 (2008).
Francia, G. et al. Long term progression and therapeutic response of visceral metastatic disease non-invasively monitored in mouse urine using β-hCG choriogonadotropin secreting tumor cell lines. Mol. Cancer Ther. 7, 3452–3459 (2008).
Fidler, I. J. & Hart, I. R. Biological diversity in metastatic neoplasms: origins and implications. Science 217, 998–1003 (1982).
Barnett, S. C. & Eccles, S. A. Studies of mammary carcinoma metastasis in a mouse model system. I: derivation and characterization of cells with different metastatic properties during tumour progression in vivo. Clin. Exp. Metastasis 2, 15–36 (1984).
Shih, I. M. et al. Assessing tumors in living animals through measurement of urinary β-human chorionic gonadotropin. Nature Med. 6, 711–714 (2000).
Hoffman, R. M. Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clin. Exp. Metastasis 26, 345–355 (2009).
Sahai, E. Illuminating the metastatic process. Nature Rev. Cancer 7, 737–749 (2007).
Kozlowski, J. M. et al. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 44, 3522–3529 (1984).
Poste, G., Doll, J., Hart, I. R. & Fidler, I. J. In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res. 40, 1636–1644 (1980).
Kerbel, R. S., Man, M. S. & Dexter, D. A model of human cancer metastasis: extensive spontaneous and artificial metastasis of a human pigmented and derived variant sublines in nude mice. J. Natl Cancer Inst. 72, 93–108 (1984).
Price, J. E., Polyzos, A., Zhang, R. D. & Daniels, L. M. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 50, 717–721 (1990).
Francia, G. et al. Comparative impact of trastuzumab and cyclophosphamide on HER-2 positive human breast cancer xenografts. Clin. Cancer Res. 15, 6358–6366 (2009).
du Manoir, J. M. et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab (Herceptin®) in human breast cancer xenografts. Clin. Cancer Res. 12, 904–916 (2006).
Man, S. et al. Antitumor and anti-angiogenic effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 62, 2731–2735 (2002).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Hudis, C. A. Trastuzumab-mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Kerbel, R. S. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science 312, 1171–1175 (2006).
Burger, R. A. et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): a Gynecologic Oncology Group study. J. Clin. Oncol. Abst. 28, LBA1 (2010).
Man, S., Munoz, R. & Kerbel, R. S. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 26, 737–747 (2007).
Gril, B. et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J. Natl Cancer Inst. 100, 1092–1103 (2008).
Warren, R. S., Yuan, H., Mati, M. R., Gillett, N. A. & Ferrara, N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J. Clin. Invest. 95, 1789–1797 (1995).
Vantyghem, S. A. et al. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 65, 3396–3403 (2005).
McCulloch, P. & George, W. D. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br. J. Cancer 59, 179–183 (1989).
Dellapasqua, S. et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer: clinical and biological activity. J. Clin. Oncol. 26, 4899–4905 (2008).
Cruz-Munoz, W., Man, S. & Kerbel, R. S. Effective treatment of advanced human melanoma metastasis in immunodeficient mice using combination metronomic chemotherapy regimens. Clin. Cancer Res. 15, 4867–4874 (2009).
Lawson, D. H. Choices in adjuvant therapy of melanoma. Cancer Control 12, 236–241 (2005).
Lin, N. U. & Winer, E. P. Brain metastases: the HER2 paradigm. Clin. Cancer Res. 13, 1648–1655 (2007).
Steeg, P. S. et al. Preclinical drug development must consider the impact on metastasis. Clin. Cancer Res. 15, 4529–4530 (2009).
Palmieri, D. et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin. Cancer Res. 15, 6148–6157 (2009).
Carbonell, W. S., Ansorge, O., Sibson, N. & Muschel, R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4, e5857 (2009).
Minn., A. J. et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Invest. 115, 44–55 (2005).
Zhang, X. H. et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78 (2009).
Welch, D. R. Technical considerations for studying cancer metastasis in vivo. Clin. Exp. Metastasis 15, 272–306 (1997).
Steeg, P. S. & Theodorescu, D. Metastasis: a therapeutic _target for cancer. Nature Clin. Pract. Oncol. 5, 206–219 (2008).
Dunn, L. K. et al. Hypoxia and TGF-β drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS ONE 4, e6896 (2009).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Price, J. E. Analyzing the metastatic phenotype. J. Cell. Biochem. 56, 16–22 (1994).
Acknowledgements
The authors would like to thank C. Cheng for her excellent secretarial assistance; L. Ellis, I. Hart, I. J. Fidler, C. Hackl and U. Emmenegger for their comments, past and present. R.S.K.'s metastasis therapy studies are supported by grants from the US National Institutes of Health (NIH)(CA-41233), Canadian Cancer Society Research Institute (CCSRI), Canadian Institutes of Health Research (CIHR) and the Ontario Institute for Cancer Research (OICR), as well as past or present sponsored research agreements with Taiho Pharmaceuticals, Tokyo, Japan, ImClone Systems, New York, GSK, Philadelphia and Pfizer, La Jolla, USA. R.S.K. holds a Tier I Canada Research Chair in Tumour Biology, Angiogenesis and Antiangiogenic Therapy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.S.K. is a consultant at Taiho Pharmaceuticals, Japan, and a member of the Scientific Advisory Board at MetronomX, USA.
Related links
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Francia, G., Cruz-Munoz, W., Man, S. et al. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer 11, 135–141 (2011). https://doi.org/10.1038/nrc3001
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3001