Abstract
The majority of genetic mutations associated with hypertrophic cardiomyopathy (HCM) occur in genes encoding sarcomeric proteins, which are expressed only in cardiomyocytes. However, some manifestations of the HCM phenotype, such as myocardial disarray, interstitial fibrosis, mitral valve abnormalities, and microvascular remodeling, indicate the involvement of other cell lineages. The link between sarcomeric gene defects and these 'extended' HCM phenotypes remains elusive. Based on novel insights provided by cardiac developmental biology, we propose that a common lineage ancestry of the diverse HCM phenotypes not involving the cardiomyocyte can be traced to the pluripotent epicardium-derived cells (EPDCs). During cardiac colonization, EPDCs differentiate into interstitial fibroblasts, coronary smooth-muscle cells, and atrioventricular endocardial cushions as mesenchymal cells. We propose that the cross-talk between healthy EPDCs and abnormally contracting cardiomyocytes might account for the diverse manifestations of HCM, by a putative mechanism of mechanotransduction leading to abnormal gene expression and differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maron, B. J. Hypertrophic cardiomyopathy: a systematic review. JAMA 287, 1308–1320 (2002).
Yacoub, M. H., Olivotto, I. & Cecchi, F. 'End-stage' hypertrophic cardiomyopathy: from mystery to model. Nat. Clin. Pract. Cardiovasc. Med. 4, 232–233 (2007).
Ashrafian, H. & Watkins, H. Reviews of translational medicine and genomics in cardiovascular disease: new disease taxonomy and therapeutic implications cardiomyopathies: therapeutics based on molecular phenotype. J. Am. Coll. Cardiol. 49, 1251–1264 (2007).
Ho, C. Y. & Seidman, C. E. A contemporary approach to hypertrophic cardiomyopathy. Circulation 113, e858–e862 (2006).
Olivotto, I. et al. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin. Proc. 83, 630–638 (2008).
Richard, P. et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107, 2227–2232 (2003).
Basso, C. et al. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum. Pathol. 31, 988–998 (2000).
Harrigan, C. J. et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am. J. Cardiol. 101, 668–673 (2008).
Klues, H. G., Maron, B. J., Dollar, A. L. & Roberts, W. C. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 85, 1651–1660 (1992).
Markwald, R. R. & Butcher, J. T. The next frontier in cardiovascular developmental biology—an integrated approach to adult disease? Nat. Clin. Pract. Cardiovasc. Med. 4, 60–61 (2007).
Olivotto, I. et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 52, 559–566 (2008).
Phadke, R. S., Vaideeswar, P., Mittal, B. & Deshpande, J. Hypertrophic cardiomyopathy: an autopsy analysis of 14 cases. J. Postgrad. Med. 47, 165–170 (2001).
Adabag, A. S. et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 51, 1369–1374 (2008).
Cecchi, F. et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 349, 1027–1035 (2003).
Yacoub, M., Onuzo, O., Riedel, B. & Radley-Smith, R. Mobilization of the left and right fibrous trigones for relief of severe left ventricular outflow obstruction. J. Thorac. Cardiovasc. Surg. 117, 126–132 (1999).
Monserrat, L. et al. Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur. Heart J. 28, 1953–1961 (2007).
Olivotto, I. et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 104, 2517–2524 (2001).
chäfers, M. et al. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ. Res. 82, 57–62 (1998).
Lie-Venema, H. et al. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. ScientificWorldJournal 7, 1777–1798 (2007).
Eisenberg, L. M. & Markwald, R. R. Cellular recruitment and the development of the myocardium. Dev. Biol. 274, 225–232 (2004).
Cai, C. L. et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 5, 877–889 (2003).
Franco, D. et al. Left and right ventricular contributions to the formation of the interventricular septum in the mouse heart. Dev. Biol. 294, 366–375 (2006).
Lie-Venema, H. et al. Periostin expression by epicardium-derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation 76, 809–819 (2008).
Perez-Pomares, J. M. et al. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Dev. Biol. 247, 307–326 (2002).
Dosdall, D. J. et al. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am. J. Physiol. Heart Circ. Physiol. 295, H883–H889 (2008).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Dahl, K. N., Ribeiro, A. J. & Lammerding, J. Nuclear shape, mechanics and mechanotransduction. Circ. Res. 102, 1307–1318 (2008).
Belus, A. et al. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J. Physiol. 586, 3639–3644 (2008).
Douglas, Y. L. et al. Pulmonary vein, dorsal atrial wall and atrial septum abnormalities in podoplanin knockout mice with disturbed posterior heart field contribution. Pediatr. Res. (in press).
Mahtab, E. A. et al. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev. Dyn. 237, 847–857 (2008).
Cai, C. L. et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 (2008).
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008).
Klaassen, S. et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation 117, 2893–2901 (2008).
Peng, X. et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc. Natl Acad. Sci. USA 105, 6638–6643 (2008).
Acknowledgements
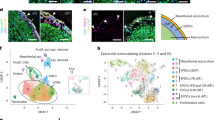
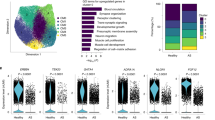
Financial support from Telethon-Italy (GGP07133) and MiUr (PRIN2006) are gratefully acknowledged. We are indebted to Dr Francesca Garbini for providing the histological images shown in Figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Olivotto, I., Cecchi, F., Poggesi, C. et al. Developmental origins of hypertrophic cardiomyopathy phenotypes: a unifying hypothesis. Nat Rev Cardiol 6, 317–321 (2009). https://doi.org/10.1038/nrcardio.2009.9
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2009.9
This article is cited by
-
Valvular heart disease and cardiomyopathy: reappraisal of their interplay
Nature Reviews Cardiology (2024)
-
Mechanisms and prognostic impact of myocardial ischaemia in hypertrophic cardiomyopathy
The International Journal of Cardiovascular Imaging (2023)
-
Altered intercellular communication and extracellular matrix signaling as a potential disease mechanism in human hypertrophic cardiomyopathy
Scientific Reports (2022)
-
Coronary arterial vasculature in the pathophysiology of hypertrophic cardiomyopathy
Pflügers Archiv - European Journal of Physiology (2019)
-
Ventricular myocardium development and the role of connexins in the human fetal heart
Scientific Reports (2017)