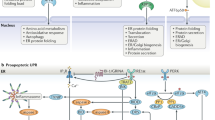
Key Points
-
Alterations in the function of the endoplasmic reticulum (ER) can result in the accumulation of unfolded or misfolded proteins, a cellular condition referred to as ER stress. ER stress engages the unfolded protein response (UPR), an adaptive reaction that reduces unfolded protein load to maintain cell viability and function.
-
Conditions of chronic or irreversible ER stress trigger cell death by apoptosis, a process that involves the activation of the canonical mitochondrial pathway, which is controlled by the B cell lymphoma 2 (BCL-2) protein family. Chronic ER stress has been linked to the occurrence of many diseases, including cancer, neurodegeneration, ischaemia and diabetes.
-
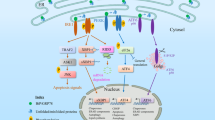
The UPR is a complex signal transduction pathway that is initiated by the activation of at least three UPR stress sensors: inositol-requiring protein 1 (IRE1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6). These sensors controls adaptive process through both transcriptional and non-transcriptional responses, affecting almost every aspect of the secretory pathway, including protein folding, ER biogenesis, ER-associated degradation (ERAD), protein entry to the ER, autophagy and secretion, among others.
-
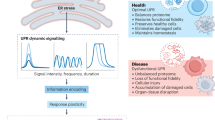
UPR signalling has distinct kinetics, intensities and downstream consequences depending on the nature and intensity of the stimuli and the cell type involved. These effects may be explained by the modulation of UPR stress sensor activity through interactions with several modulators and adaptor proteins, which possibly results in the assembly of dynamic signalling platforms that have been referred to as 'UPRosomes'.
-
Recent advances in the UPR field indicate that this pathway has important functions in many physiological processes that are not directly related to protein folding. For example, UPR signalling modules crosstalk with signalling pathways that are key in the control of lipid and energy metabolism, innate immunity and cell differentiation programmes.
Abstract
Protein-folding stress at the endoplasmic reticulum (ER) is a salient feature of specialized secretory cells and is also involved in the pathogenesis of many human diseases. ER stress is buffered by the activation of the unfolded protein response (UPR), a homeostatic signalling network that orchestrates the recovery of ER function, and failure to adapt to ER stress results in apoptosis. Progress in the field has provided insight into the regulatory mechanisms and signalling crosstalk of the three branches of the UPR, which are initiated by the stress sensors protein kinase RNA-like ER kinase (PERK), inositol-requiring protein 1α (IRE1α) and activating transcription factor 6 (ATF6). In addition, novel physiological outcomes of the UPR that are not directly related to protein-folding stress, such as innate immunity, metabolism and cell differentiation, have been revealed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev. Mol. Cell Biol. 8, 519–529 (2007).
Kozutsumi, Y., Segal, M., Normington, K., Gething, M. J. & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988).
Schroder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Hetz, C., Martinon, F., Rodriguez, D. & Glimcher, L. H. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 91, 1219–1243 (2011).
Woehlbier, U. & Hetz, C. Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem. Sci. 36, 329–337 (2011).
Rutkowski, D. T. & Hegde, R. S. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 189, 783–794 (2010).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Han, D. et al. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 (2009).
Hollien, J. et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331 (2009).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Kroemer, G., Marino, G. & Levine, B. Autophagy and the integrated stress response. Mol. Cell 40, 280–293 (2010).
Kang, S. W. et al. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127, 999–1013 (2006).
Oyadomari, S. et al. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell 126, 727–739 (2006).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Lee, K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001). References 15–17 identify XBP1 mRNA as a substrate of IRE1α.
Lee, A. H., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459 (2003).
Acosta-Alvear, D. et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 27, 53–66 (2007).
Asada, R., Kanemoto, S., Kondo, S., Saito, A. & Imaizumi, K. The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J. Biochem. 149, 507–518 (2011).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Ameri, K. & Harris, A. L. Activating transcription factor 4. Int. J. Biochem. Cell Biol. 40, 14–21 (2008).
Behrman, S., Acosta-Alvear, D. & Walter, P. A CHOP-regulated microRNA controls rhodopsin expression. J. Cell Biol. 192, 919–927 (2011).
Tabas, I. & Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature Cell Biol. 13, 184–190 (2011).
Tait, S. W. & Green, D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Rev. Mol. Cell Biol. 11, 621–632 (2010).
Hetz, C. & Glimcher, L. H. Fine-tuning of the unfolded protein response: assembling the IRE1α interactome. Mol. Cell 35, 551–561 (2009).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Nassif, M., Matus, S., Castillo, K. & Hetz, C. Amyotrophic lateral sclerosis pathogenesis: a journey through the secretory pathway. Antioxid. Redox Signal. 13, 1955–1989 (2010).
Nakajima, S. et al. Selective abrogation of BiP/GRP78 blunts activation of NF-κB through the ATF6 branch of the UPR: involvement of C/EBPβ and mTOR-dependent dephosphorylation of Akt. Mol. Cell. Biol. 31, 1710–1718 (2011).
Kimata, Y. & Kohno, K. Endoplasmic reticulum stress-sensing mechanisms in yeast and mammalian cells. Curr. Opin. Cell Biol. 23, 135–142 (2011).
Li, H., Korennykh, A. V., Behrman, S. L. & Walter, P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl Acad. Sci. USA 107, 16113–16118 (2010).
Korennykh, A. V. et al. The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 (2009).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2, 326–332 (2000).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Schindler, A. J. & Schekman, R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc. Natl Acad. Sci. USA 106, 17775–17780 (2009).
Hong, M. et al. Underglycosylation of ATF6 as a novel sensing mechanism for activation of the unfolded protein response. J. Biol. Chem. 279, 11354–11363 (2004).
Nadanaka, S., Okada, T., Yoshida, H. & Mori, K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol. Cell. Biol. 27, 1027–1043 (2007).
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005).
Gardner, B. M. & Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 (2011). References 41 and 42 depict a direct model for unfolded protein recognition by Ire1.
Oikawa, D., Kimata, Y., Kohno, K. & Iwawaki, T. Activation of mammalian IRE1α upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp. Cell Res. 315, 2496–2504 (2009).
Zhou, J. et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl Acad. Sci. USA 103, 14343–14348 (2006).
Yoshida, H. et al. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell 4, 265–271 (2003).
DuRose, J. B., Tam, A. B. & Niwa, M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol. Biol. Cell 17, 3095–3107 (2006).
Maiuolo, J., Bulotta, S., Verderio, C., Benfante, R. & Borgese, N. Selective activation of the transcription factor ATF6 mediates endoplasmic reticulum proliferation triggered by a membrane protein. Proc. Natl Acad. Sci. USA 108, 7832–7837 (2011).
Lee, A. H., Heidtman, K., Hotamisligil, G. S. & Glimcher, L. H. Dual and opposing roles of the unfolded protein response regulated by IRE1α and XBP1 in proinsulin processing and insulin secretion. Proc. Natl Acad. Sci. USA 108, 8885–8890 (2011).
Lipson, K. L., Ghosh, R. & Urano, F. The role of IRE1α in the degradation of insulin mRNA in pancreatic β-cells. PLoS ONE 3, e1648 (2008).
Iqbal, J. et al. IRE1β inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 7, 445–455 (2008).
Liu, C. Y., Schroder, M. & Kaufman, R. J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 275, 24881–24885 (2000).
Lin, J. H. et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 (2007).
Lin, J. H., Li, H., Zhang, Y., Ron, D. & Walter, P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 4, e4170 (2009).
Pincus, D. et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 (2010).
Rubio, C. et al. Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J. Cell Biol. 193, 171–184 (2011).
Chawla, A., Chakrabarti, S., Ghosh, G. & Niwa, M. Attenuation of yeast UPR is essential for survival and is mediated by IRE1 kinase. J. Cell Biol. 193, 41–50 (2011).
Rutkowski, D. T. et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 (2006).
Hetz, C. et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312, 572–576 (2006).
Gupta, S. et al. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1α–XBP1 signaling through a physical interaction. PLoS Biol. 8, e1000410 (2010).
Gu, F. et al. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J. Biol. Chem. 279, 49689–49693 (2004).
Luo, D. et al. AIP1 is critical in transducing IRE1-mediated endoplasmic reticulum stress response. J. Biol. Chem. 283, 11905–11912 (2008). References 57, 58 and 60 give the first examples of specific IRE1α cofactors that tune UPR signalling.
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Oono, K. et al. JAB1 participates in unfolded protein responses by association and dissociation with IRE1. Neurochem. Int. 45, 765–772 (2004).
Lisbona, F. et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1α. Mol. Cell 33, 679–691 (2009). References 52, 54, 55 and 63 provide insights into the attenuation of IRE1 signalling.
Bailly-Maitre, B. et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 103, 2809–2814 (2006).
Bailly-Maitre, B. et al. Hepatic Bax inhibitor-1 inhibits IRE1α and protects from obesity-associated insulin resistance and glucose intolerance. J. Biol. Chem. 285, 6198–6207 (2010).
Rong, J. et al. BAR, an endoplasmic reticulum-associated E3 ubiquitin ligase, modulates BI-1 protein stability and function in ER stress. J. Biol. Chem. 286, 1453–1463 (2010).
Kato, H. et al. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ. 22 Jul 2011 (doi: 10.1038/cdd.2011.98).
Wiseman, R. L. et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol. Cell 38, 291–304.
Korennykh, A. V. et al. Cofactor-mediated conformational control in the bifunctional kinase/RNase Ire1. BMC Biol. 9, 48 (2011).
Ishiwata-Kimata, Y. et al. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum-stress sensor Ire1 by different manners. Mol. Biol. Cell 22, 3520–3532 (2011).
Bouchecareilh, M., Higa, A., Fribourg, S., Moenner, M. & Chevet, E. Peptides derived from the bifunctional kinase/RNase enzyme IRE1α modulate IRE1α activity and protect cells from endoplasmic reticulum stress. FASEB J. 25, 3115–3129 (2011).
van Huizen, R., Martindale, J. L., Gorospe, M. & Holbrook, N. J. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2α signaling. J. Biol. Chem. 278, 15558–15564 (2003).
Yan, W. et al. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl Acad. Sci. USA 99, 15920–15925 (2002).
Ni, M., Zhou, H., Wey, S., Baumeister, P. & Lee, A. S. Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP. PLoS ONE 4, e6868 (2009).
Bollo, M. et al. Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS ONE 5, e11925 (2010).
Fonseca, S. G. et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest. 120, 744–755 (2010).
Yoshida, H. et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20, 6755–6767 (2000).
Luo, R., Lu, J. F., Hu, Q. & Maity, S. N. CBF/NF-Y controls endoplasmic reticulum stress induced transcription through recruitment of both ATF6(N) and TBP. J. Cell Biochem. 104, 1708–1723 (2008).
Li, M. et al. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 20, 5096–5106 (2000).
Sato, Y., Nadanaka, S., Okada, T., Okawa, K. & Mori, K. Luminal domain of ATF6 alone is sufficient for sensing endoplasmic reticulum stress and subsequent transport to the Golgi apparatus. Cell Struct. Funct. 36, 35–47 (2011).
Yanagitani, K. et al. Cotranslational _targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol. Cell 34, 191–200 (2009).
Yanagitani, K., Kimata, Y., Kadokura, H. & Kohno, K. Translational pausing ensures membrane _targeting and cytoplasmic splicing of XBP1u mRNA. Science 331, 586–589 (2011). References 81 and 82 report a mechanism for _targeting XBP1 mRNA to IRE1α for splicing.
Park, S. W. et al. The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nature Med. 16, 429–437 (2010).
Winnay, J. N., Boucher, J., Mori, M. A., Ueki, K. & Kahn, C. R. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nature Med. 16, 438–445 (2010).
Lee, J. et al. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nature Med. 17, 1251–1260 (2011).
Wang, F. M. & Ouyang, H. J. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem. J. 433, 245–252 (2010).
Chen, H. & Qi, L. SUMO modification regulates the transcriptional activity of XBP1. Biochem. J. 429, 95–102 (2010).
Yoshida, H., Oku, M., Suzuki, M. & Mori, K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J. Cell Biol. 172, 565–575 (2006).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Boyce, M. et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Tsaytler, P., Harding, H. P., Ron, D. & Bertolotti, A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 (2011).
Teske, B. F. et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol. Biol. Cell 22, 4390–4405 (2011).
Martinon, F. & Glimcher, L. H. Regulation of innate immunity by signaling pathways emerging from the endoplasmic reticulum. Curr. Opin. Immunol. 23, 35–40 (2011).
Martinon, F., Chen, X., Lee, A.-H. & Glimcher, L. H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature Immunol. 11, 411–418 (2010).
Woo, C. W. et al. Adaptive suppression of the ATF4–CHOP branch of the unfolded protein response by toll-like receptor signalling. Nature Cell Biol. 11, 1473–1480 (2009).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Lipson, K. L. et al. Regulation of insulin biosynthesis in pancreatic β cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 4, 245–254 (2006).
Qiu, Y. et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1α activation in pancreatic β cells. Sci. Signal. 3, ra7 (2010).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Fu, S. et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473, 528–531 (2011).
Reimold, A. M. et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 14, 152–157 (2000).
Iwakoshi, N. N. et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature Immunol. 4, 321–329 (2003).
Lee, A. H., Chu, G. C., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005).
Iwawaki, T., Akai, R. & Kohno, K. IRE1α disruption causes histological abnormality of exocrine tissues, increase of blood glucose level, and decrease of serum immunoglobulin level. PLoS ONE 5, e13052 (2010).
Huh, W. J. et al. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 139, 2038–2049 (2010).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Hu, C., Dougan, S., McGehee, A., Love, J. & Ploegh, H. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. (2009).
Zhang, K. et al. The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest. 115, 268–281 (2005).
Tsang, K. Y. et al. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol. 5, e44 (2007).
Lee, A.-H., Scapa, E., Cohen, D. & Glimcher, L. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496 (2008).
Yamamoto, K. et al. Induction of liver steatosis and lipid droplet formation in ATF6α-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol. Biol. Cell 21, 2975–2986 (2010).
Zhang, K. et al. The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J. 30, 1357–1375 (2011).
Vecchi, C. et al. ER stress controls iron metabolism through induction of hepcidin. Science 325, 877–880 (2009).
Zhou, Y. et al. Regulation of glucose homeostasis through a XBP-1–FoxO1 interaction. Nature Med. 17, 356–365 (2011).
Henis-Korenblit, S. et al. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc. Natl Acad. Sci. USA 107, 9730–9735 (2010).
Wang, Y., Vera, L., Fischer, W. H. & Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460, 534–537 (2009).
Hayashi, A. et al. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J. Biol. Chem. 282, 34525–34534 (2007).
Bommiasamy, H. & Popko, B. Animal models in the study of the unfolded protein response. Meth. Enzymol. 491, 91–109 (2011).
Hetz, C. & Glimcher, L. H. Protein homeostasis networks in physiology and disease. Curr. Opin. Cell Biol. 23, 123–125 (2011).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001). Provides one of the first clues to the physiological function of the mammalian UPR.
Iwawaki, T., Akai, R., Yamanaka, S. & Kohno, K. Function of IRE1α in the placenta is essential for placental development and embryonic viability. Proc. Natl Acad. Sci. USA 106, 16657–16662 (2009).
Gass, J. N., Jiang, H. Y., Wek, R. C. & Brewer, J. W. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol. Immunol. 45, 1035–1043 (2008).
Yang, X. et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell 117, 387–398 (2004).
Tanaka, T. et al. _targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes Cells 3, 801–810 (1998).
Matus, S., Glimcher, L. H. & Hetz, C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr. Opin. Cell Biol. 23, 239–252 (2011).
Saxena, S., Cabuy, E. & Caroni, P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nature Neurosci. 12, 627–636 (2009).
Wang, L., Popko, B. & Roos, R. P. The unfolded protein response in familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 20, 1008–1015 (2011).
Hetz, C. et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 23, 2294–2306 (2009).
Hetz, C. et al. The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ. 14, 1386–1389 (2007).
Nishitoh, H. et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by _targeting Derlin-1. Genes Dev. 22, 1451–1464 (2008).
Acknowledgements
I apologize to all colleagues whose work could not be cited owing to space limitations. I thank A. Couve, U. Woehlbier and A. Glavic for constructive comments, C. Wirth for editing and D. Rodriguez for input into the initial figure design. This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile, grant 1100176, Fondo de Investigación Avanzado en Areas Prioritarias (FONDAP), Chile, grant 15010006, Millennium Institute grant P09-015-F the Muscular Dystrophy Association, the Michael J. Fox Foundation for Parkinson Research, the Alzheimer's Association and the North American Spine Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- RIDD
-
(Regulated IRE1-dependent decay). The degradation of a subset of mRNAs encoding for proteins located in the endoplasmic reticulum, possibly through the activation of the RNase domain of inositol-requiring 1 (IRE1).
- ERAD
-
(Endoplasmic reticulum-associated degradation). A pathway along which misfolded proteins are transported from the ER to the cytosol for proteasomal degradation.
- Autophagy
-
A survival pathway that is classically linked to the adaptation to nutrient starvation through the recycling of cytosolic components by lysosome-mediated degradation. In cells undergoing endoplasmic reticulum stress, autophagy may serve as a mechanism to eliminate damaged organelles and aggregated proteins.
- Pancreatic β-cells
-
Cells in the pancreas that make and secrete insulin to respond to glucose fluctuations.
- UPRosome
-
A signalling platform assembled at the level of inositol-requiring protein 1α that controls the kinetics and amplitude of downstream unfolded protein response (UPR) signalling responses. The UPRosome also orchestrates crosstalk between the UPR and other signalling pathways through the recruitment of different adaptor proteins.
- Exocrine pancreas
-
A type of pancreatic tissue that has ducts arranged in clusters called acini. Cells secrete into the lumen of an acinus a series of enzymes and molecules related to digestion, including trypsinogen, lipase, amylase and ribonuclease.
- Endocrine pancreas
-
The part of the pancreas that acts as an endocrine gland, consisting of the islets of Langerhans, which contain β-cells. Theses cells secrete insulin and other hormones.
Rights and permissions
About this article
Cite this article
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13, 89–102 (2012). https://doi.org/10.1038/nrm3270
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3270
This article is cited by
-
Melatonin attenuates diabetic cardiomyopathy by increasing autophagy of cardiomyocytes via regulation of VEGF-B/GRP78/PERK signaling pathway
Cardiovascular Diabetology (2024)
-
Glucose dysregulation in antipsychotic-naive first-episode psychosis: in silico exploration of gene expression signatures
Translational Psychiatry (2024)
-
FBXO5-mediated RNF183 degradation prevents endoplasmic reticulum stress-induced apoptosis and promotes colon cancer progression
Cell Death & Disease (2024)
-
A novel bystander effect in tamoxifen treatment: PPIB derived from ER+ cells attenuates ER− cells via endoplasmic reticulum stress-induced apoptosis
Cell Death & Disease (2024)
-
Reversibility of Endoplasmic Reticulum Stress Markers During Long-Term Glucose Starvation in Astrocytes
Journal of Molecular Neuroscience (2024)